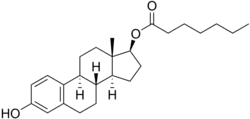
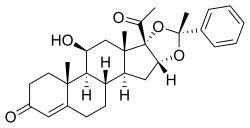
Estradiol enantate/algestone acetophenide
Estradiol enantate/algestone acetophenide, also known as estradiol enantate/dihydroxyprogesterone acetophenide (E2-EN/DHPA) and sold under the brand names Perlutal and Topasel among others, is a form of combined injectable birth control which is used to prevent pregnancy.[1][2][3] It contains estradiol enantate (E2-EN), an estrogen, and algestone acetophenide (dihydroxyprogesterone acetophenide; DHPA), a progestin.[1][2][3] The medication is given once a month by injection into muscle.[1][2][3]
 | |
 | |
| Combination of | |
|---|---|
| Estradiol enantate | Estrogen |
| Algestone acetophenide | Progestogen |
| Clinical data | |
| Trade names | Perlutal, Topasel, Unalmes, Yectames, many others |
| Other names | Estradiol enantate/dihydroxyprogesterone acetophenide; E2-EN/DHPA |
| Routes of administration | Intramuscular injection |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
E2-EN/DHPA is used widely throughout Latin America, is also marketed in Hong Kong, and was previously available in Portugal and Spain as well but was discontinued in these countries.[1][2][3][4]
Medical uses
E2-EN/DHPA is used in combination as a once-monthly combined injectable contraceptive to prevent pregnancy in women in Latin America and Hong Kong.[5][3][6] E2-EN/DHPA has been said to be used by "travestis" (a term for transgender women in some cultures, especially in South America) as a means of feminizing hormone therapy as well.[7]
Available forms
The following forms of E2-EN/DHPA are or have been available for use:[3][8][9][10][5]
- E2-EN 10 mg and DHPA 150 mg (brand names Perlutal, Topasel, many others)
- E2-EN 5 mg and DHPA 75 mg (brand names Anafertin, Patector NF, Yectames)
- E2-EN 10 mg and DHPA 120 mg (brand names Unalmes, Yectuna)
- E2-EN 10 mg and DHPA 75 mg (brand name Ova Repos; discontinued)
A 6 mg E2-EN and 90 mg DHPA formulation was also studied, but was never marketed.[11][12][13] The combination of E2-EN and DHPA has also been studied at other doses ranging from 5 to 50 mg E2-EN and 75 to 200 mg DHPA.[14]
Pharmacology
Pharmacology
Clinical studies have found that, on the basis of endometrial changes, E2-EN/DHPA appears, at the doses used, to be an estrogen-dominant combination.[15]
Pharmacokinetics
By intramuscular injection, the elimination half-life of E2-EN has been found to be 5.6 to 7.5 days, while the half-life of algestone acetophenide and its metabolites has been found to be 24 days.[16][17][18][2] Following a single injection, E2-EN and DHPA are detectable in the circulation for up to 30 to 60 days.[19][2]
History
E2-EN/DHPA was first studied as a combined injectable contraceptive in 1964.[10] It was developed by Squibb under the developmental code name and tentative brand name Deladroxate for potential use as a combined injectable contraceptive in the United States.[20][2] Due to toxicological findings of DHPA of pituitary hyperplasia in rats, mammary tumors in beagle dogs, and "uterine swellings" in animals, as well as concerns about possible accumulation of DHPA, Squibb discontinued the development of E2-EN/DHPA in the late 1960s.[20][10][21][2] Subsequently, in 1973, a pharmacokinetic study of E2-EN/DHPA in women generated concerns about potential accumulation of E2-EN with once-monthly use as well.[20][10][2] In spite of these concerns however, no toxicity or tumorigenicity has been observed with E2-EN/DHPA in humans in extensive clinical studies, and there are doubts about the relevance of the animal findings to humans.[21][10][2] In addition, only very limited accumulation of E2-EN has been found to occur with the preparation.[2]
Manufacturers in other countries, including EuroPharma, Farmitalia, and Promeco, resumed development of E2-EN/DHPA following the discontinuation of its development by Squibb, and introduced it for clinical use as a combined injectable contraceptive, under the brand names Perlutal and Topasel, in Spain and Latin America in the 1970s.[20][15][10] It was one of only two combined injectable contraceptives to have been marketed by 1976, and was one of only three combined injectable contraceptives with considerable clinical experience by 1976.[20][15] The others were estradiol valerate/hydroxyprogesterone caproate (EV/OHPC; brand name Injectable No. 1), which had been marketed in China, and estradiol cypionate/medroxyprogesterone acetate (EC/MPA; code name Cyclo-Provera), which was still experimental by 1976 and did not become formally available for clinical use until the 1990s.[15][10] By 1994, at which point EC/MPA (brand names Cyclofem and later Lunelle) and estradiol valerate/norethisterone enantate (EV/NETE; brand name Mesigyna) had been introduced, E2-EN/DHPA had been in use for many years.[15][10]
E2-EN/DHPA and EV/OHPC have been referred to as first-generation combined injectable contraceptives, while EC/MPA and EV/NETE have been referred to as second-generation combined injectable contraceptives.[20]
Society and culture
Brand names
E2-EN has been marketed under a wide variety of brand names.[4][22][23][24][3][25][9][26][10][5][1] It has been marketed in a few different preparations, with varying doses of E2-EN and DHPA.[9][3][25][8][10][5][1] These formulations all have different brand names, which include the following († = discontinued):[4][22][23][24][8][9][3][25][5][27]
- E2-EN 10 mg / DHPA 150 mg: Acefil, Agurin†, Atrimon†, Ciclomes, Ciclovar, Ciclovular, Cicnor†, Clinomin, Cycloven, Daiva, Damix, Deprans, Deproxone, Exuna, Ginestest, Ginoplan†, Gynomes, Horprotal, Listen, Luvonal, Neogestar, Neolutin, Nomagest, Nonestrol, Normagest, Normensil, Novular, Oterol, Ovoginal, Patector, Patectro, Perludil, Perlumes, Perlutal, Perlutale, Perlutan, Perlutin, Perlutin-Unifarma, Permisil, Preg-Less, Pregnolan, Progestrol†, Protegin, Proter, Seguralmes, Synovular, Topasel, Unigalen, Uno-Ciclo, and Vagital.
- E2-EN 10 mg / DHPA 120 mg: Anafertin†, Patector NF, and Yectames.
- E2-EN 5 mg / DHPA 75 mg: Unalmes and Yectuna.
- E2-EN 10 mg / DHPA 75 mg: Ova Repos†.
- Unsorted: Evitas†, Femineo†, and Primyfar†.
The combination of E2-EN 10 mg and DHPA 150 mg was developed under the developmental brand name Deladroxate, but this brand name was never used commercially.[10][5]
Availability
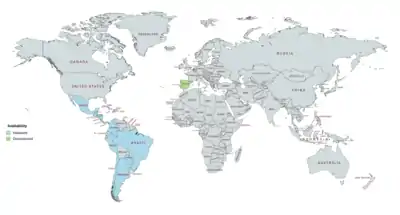
DHPA has been marketed in combination with estradiol enantate (E2-EN) as a combined injectable contraceptive in at least 19 countries, mostly in Latin America.[3][25][9][26][4][22][23][24] A few different preparations, with varying doses of E2-EN and DHPA and varying availability, have been introduced.[9][3][25][8][10][5][1] These formulations have the following approval and availability († = discontinued in this country):[4][22][23][24][8][9][3][25][5]
- E2-EN 10 mg / DHPA 150 mg: at least 19 countries, including Argentina, Belize, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Hong Kong, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal†, and Spain†.
- E2-EN 10 mg / DHPA 120 mg: at least 3 countries, including Brazil†, Chile, and Paraguay.
- E2-EN 5 mg / DHPA 75 mg: at least 9 countries, including Costa Rica, the Dominican Republic, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Spain†.
Usage
E2-EN/DHPA is the most widely used combined injectable contraceptive in Latin America.[28] It was estimated in 1995 that E2-EN/DHPA was used as a combined injectable contraceptive in Latin America by at least 1 million women.[9] However, combined injectable contraceptives like E2-EN/DHPA are unlikely to constitute a large proportion of contraceptive use in the countries in which they are available.[9]
See also
References
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- "Micromedex Products: Please Login".
- Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 (Suppl 1): S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- Rowlands S (January 2009). "New technologies in contraception" (PDF). BJOG. 116 (2): 230–239. doi:10.1111/j.1471-0528.2008.01985.x. PMID 19076955. S2CID 3415547.
- Kulick D (12 January 2009). "Becoming a Travesti". Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes. University of Chicago Press. pp. 64–66. ISBN 978-0-226-46101-4.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). "Annex 2: Compositions of Oral and Injectable Estrogen-Progestogen Contraceptives". Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–433, 467. ISBN 978-92-832-1291-1.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- d'Arcangues C, Snow RC (1999). "Injectable Contraceptives". In Rabe T, Runnebaum B (eds.). Fertility Control — Update and Trends. pp. 121–149. doi:10.1007/978-3-642-86696-8_6. ISBN 978-3-642-86698-2.
- Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, Yazlle ME, de Souza RN, Pinho Neto JS, Leal Wde B, Leal C, Hippolito SB, Abranches AD (March 1997). "Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate". Contraception. 55 (3): 175–81. doi:10.1016/S0010-7824(97)00018-8. PMID 9115007.
- Coutinho EM, Spinola P, Tomaz G, Morais K, Nassar de Souza R, Sabino Pinho Neto J, de Barros Leal W, Bomfim Hippolito S, D'Aurea Abranches A (April 2000). "Efficacy, acceptability, and clinical effects of a low-dose injectable contraceptive combination of dihydroxyprogesterone acetophenide and estradiol enanthate". Contraception. 61 (4): 277–80. doi:10.1016/S0010-7824(00)00099-8. PMID 10899484.
- Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–98. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- "Bula do Algestona Acetofenida + Enantato de Estradiol". Consulta Remédios. Archived from the original on 2018-09-18. Retrieved 2018-09-18.
- Wiemeyer JC, Fernandez M, Moguilevsky JA, Sagasta CL (November 1986). "Pharmacokinetic studies of estradiol enantate in menopausic women". Arzneimittel-Forschung. 36 (11): 1674–1677. PMID 3814225.
- Jarquín González JD, Elda de Aguirre L, Rodríguez C, Abrego de Aguilar M, Carrillo F, León DA, et al. (September 1996). "Dihydroxyprogesterone acetophenide 150 mg + estradiol enantate 10 mg as monthly injectable contraceptives". Advances in Contraception. 12 (3): 213–225. doi:10.1007/BF01849664. PMID 8910663. S2CID 2522426.
- Gual C, Pérez-Palacios G, Perez AE, Ruiz MR, Solis J, Cervantes A, Iramain C, Schreiber EC (1973). "Metabolic fate of a long-acting injectable estrogen-progestogen contraceptive 1,2". Contraception. 7 (4): 271–287. doi:10.1016/0010-7824(73)90145-5. ISSN 0010-7824.
- del Carmen Cravioto M (15 September 1995). "Combined injectable preparations an introduction as a new method". In Bringer J, Hedon B (eds.). Fertility and Sterility: A Current Overview. CRC Press. pp. 47–. ISBN 978-1-85070-694-6.
- Skegg DC (May 1994). "Monthly combined injectable contraceptives and neoplasia". Contraception. 49 (5): 435–9. doi:10.1016/0010-7824(94)90002-7. PMID 8045130.
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- "Algestone - Drugs.com". Archived from the original on 2018-09-18. Retrieved 2018-09-18.
- "Progestin Oral, Parenteral, Vaginal Advanced Patient Information".
- Senanayake P, Potts M (14 April 2008). "Hormonal Contraception". Atlas of Contraception (Second ed.). CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- Lähteenmäki P (6 December 2012). "Intrauterine Hormone-Releasing Systems". In Rabe T, Runnebaum B (eds.). Fertility Control — Update and Trends: Update and Trends. Springer Science & Business Media. pp. 183–. ISBN 978-3-642-86696-8.
Two additional monthly, combined injectable methods warrant mention. Deladroxate (commercially labelled as Perlutan, Topasel, Agurin, Horprotal and Uno-Ciclo in various countries), is a combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate, and is available in many Latin American countries and Spain. The method is highly effective, without a single pregnancy reported in large clinical trials (Koetsawang 1994). Although available since the 1960s, the method has not been studied as extensively as Cyclofem or Mesigyna. The original manufacturer withdrew support due to toxicological concerns with dihydroxyprogesterone acetophenide, and clinical evaluations continue to be published. A recent dose-finding trial compared the standard available dose of 150/10 with a lower dose of 90/6, and concluded the lower dose was equally effective (Coutinho et al., 1997).
- Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". Cochrane Database Syst Rev. 3: CD004568. doi:10.1002/14651858.CD004568.pub3. PMC 6513542. PMID 23641480.
- Speroff L, Fritz MA (2005). "Long-Acting Methods of Contraception". Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 969–. ISBN 978-0-7817-4795-0.