Lithium fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts.[4] Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.229 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiF | |
| Molar mass | 25.939(2) g/mol |
| Appearance | White powder or colorless hygroscopic crystals |
| Density | 2.635 g/cm3 |
| Melting point | 845 °C (1,553 °F; 1,118 K) |
| Boiling point | 1,676 °C (3,049 °F; 1,949 K) |
| 0.127 g/(100 mL) (18 °C) 0.134 g/(100 mL) (25 °C) | |
Solubility product (Ksp) |
1.84×10−3[1] |
| Solubility | soluble in HF insoluble in alcohol |
| −10.1·10−6 cm3/mol | |
Refractive index (nD) |
1.3915 |
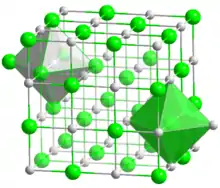
| Structure | |
| Face-centered cubic | |
a = 403.51 pm | |
| Linear | |
| Thermochemistry | |
Heat capacity (C) |
1.507 J/(g·K) |
Std molar entropy (S⦵298) |
35.73 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
-616 kJ/mol |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H301, H315, H319, H335[2] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
143 mg/kg (oral, rat)[3] |
| Related compounds | |
Other anions |
Lithium chloride Lithium bromide Lithium iodide Lithium astatide |
Other cations |
Sodium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride Francium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Manufacturing
LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride.[5]
Applications
Precursor to lithium hexafluorophosphate for batteries
Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make lithium hexafluorophosphate Li[PF6], an ingredient in lithium ion battery electrolyte.
In molten salts
Fluorine is produced by the electrolysis of molten potassium bifluoride. This electrolysis proceeds more efficiently when the electrolyte contains a few percent of LiF, possibly because it facilitates formation of an Li-C-F interface on the carbon electrodes.[4] A useful molten salt, FLiNaK, consists of a mixture of LiF, together with sodium fluoride and potassium fluoride. The primary coolant for the Molten-Salt Reactor Experiment was FLiBe; 2LiF·BeF2 (66 mol% of LiF, 33 mol% of BeF2).
Optics
Because of the large band gap for LiF, its crystals are transparent to short wavelength ultraviolet radiation, more so than any other material. LiF is therefore used in specialized optics for the vacuum ultraviolet spectrum.[6] (See also magnesium fluoride.) Lithium fluoride is used also as a diffracting crystal in X-ray spectrometry.
Radiation detectors
It is also used as a means to record ionizing radiation exposure from gamma rays, beta particles, and neutrons (indirectly, using the 6
3Li
(n,alpha) nuclear reaction) in thermoluminescent dosimeters. 6LiF nanopowder enriched to 96% has been used as the neutron reactive backfill material for microstructured semiconductor neutron detectors (MSND).[7]
Nuclear reactors
Lithium fluoride (highly enriched in the common isotope lithium-7) forms the basic constituent of the preferred fluoride salt mixture used in liquid-fluoride nuclear reactors. Typically lithium fluoride is mixed with beryllium fluoride to form a base solvent (FLiBe), into which fluorides of uranium and thorium are introduced. Lithium fluoride is exceptionally chemically stable and LiF/BeF2 mixtures (FLiBe) have low melting points (360 to 459 °C or 680 to 858 °F) and the best neutronic properties of fluoride salt combinations appropriate for reactor use. MSRE used two different mixtures in the two cooling circuits.
Cathode for PLED and OLEDs
Lithium fluoride is widely used in PLED and OLED as a coupling layer to enhance electron injection. The thickness of the LiF layer is usually around 1 nm. The dielectric constant (or relative permittivity, ε) of LiF is 9.0.[8]
Natural occurrence
Naturally occurring lithium fluoride is known as the extremely rare mineral griceite.[9]
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- "Lithium fluoride - Product Specification Sheet". Sigma-Aldrich. Merck KGaA. Retrieved 1 Sep 2019.
- "Lithium fluoride". Toxnet. NLM. Archived from the original on 12 August 2014. Retrieved 10 Aug 2014.
- Aigueperse J, Mollard P, Devilliers D, et al. (2005). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 9783527303854.
- Bellinger SL, Fronk RG, McNeil WJ, et al. (2012). "Improved High Efficiency Stacked Microstructured Neutron Detectors Backfilled With Nanoparticle 6LiF". IEEE Trans. Nucl. Sci. 59 (1): 167–173. Bibcode:2012ITNS...59..167B. doi:10.1109/TNS.2011.2175749. S2CID 19657691.
- "Lithium Fluoride (LiF) Optical Material". Crystran 19. 2012.
- McGregor DS, Bellinger SL, Shultis JK (2013). "Present status of microstructured semiconductor neutron detectors". Journal of Crystal Growth. 379: 99–110. Bibcode:2013JCrGr.379...99M. doi:10.1016/j.jcrysgro.2012.10.061. hdl:2097/16983.
- Andeen C, Fontanella J, Schuele D (1970). "Low-Frequency Dielectric Constant of LiF, NaF, NaCl, NaBr, KCl, and KBr by the Method of Substitution". Phys. Rev. B. 2 (12): 5068–73. Bibcode:1970PhRvB...2.5068A. doi:10.1103/PhysRevB.2.5068.
- "Griceite mineral information and data". Mindat.org. Archived from the original on 7 March 2014. Retrieved 22 Jan 2014.
