Arbovirus
Arbovirus is an informal name for any virus that is transmitted by arthropod vectors. The term arbovirus is a portmanteau word (arthropod-borne virus).[1] Tibovirus (tick-borne virus) is sometimes used to more specifically describe viruses transmitted by ticks, a superorder within the arthropods.[2] Arboviruses can affect both animals (including humans) and plants.[3] In humans, symptoms of arbovirus infection generally occur 3–15 days after exposure to the virus and last three or four days. The most common clinical features of infection are fever, headache, and malaise, but encephalitis and viral hemorrhagic fever may also occur.[4]
| Arbovirus infection | |
|---|---|
 | |
| Tissue infected with the Rift Valley fever virus | |
| Specialty | Infectious disease |
Signs and symptoms
The incubation period – the time between when infection occurs and when symptoms appear – varies from virus to virus, but is usually limited between 2 and 15 days for arboviruses.[5] The majority of infections, however, are asymptomatic.[6] Among cases in which symptoms do appear, symptoms tend to be non-specific, resembling a flu-like illness, and are not indicative of a specific causative agent. These symptoms include fever, headache, malaise, rash and fatigue. Rarely, vomiting and hemorrhagic fever may occur. The central nervous system can also be affected by infection, as encephalitis and meningitis are sometimes observed.[7] Prognosis is good for most people, but is poor in those who develop severe symptoms, with up to a 20% mortality rate in this population depending on the virus. The very young, elderly, pregnant women, and people with immune deficiencies are more likely to develop severe symptoms.
| Arbovirus | Disease(s) | Incubation period | Symptoms | Duration of symptoms | Complications | Case fatality rate | Vector(s) | Primary host(s) | Geographic distribution | Does infection provide lifelong immunity? |
|---|---|---|---|---|---|---|---|---|---|---|
| Dengue virus | Dengue fever | 3–14 days | Asymptomatic in most cases; fever, headache, rash, muscle, and joint pains | 7–10 days | Shock, internal bleeding, and organ damage | <1% with treatment, 1–5% without; about 25% in severe cases | Aedes mosquitoes, especially Aedes aegypti | Humans | Near the equator globally | Varies[note 1] |
| Japanese encephalitis virus | Japanese encephalitis | 5–15 days | Asymptomatic in most cases; fever, headache, fatigue, nausea, and vomiting | Encephalitis, seizures, paralysis, coma, and long-term brain damage | 20–30% in encephalitis cases | Culex mosquitoes, especially Culex tritaeniorhynchus | Domestic pigs and wading birds | Southeast and East Asia | Yes | |
| Rift Valley fever virus | Rift Valley fever | 2–6 days | Fever, headache, myalgia and liver abnormalities | 4–7 days | Hemorrhagic fever, meningoencephalitis | 1% in humans; in pregnant livestock, 100% fatality rate for fetuses | Culex tritaeniorhynchus and Aedes vexans | Micropteropus pusillus and Hipposideros abae | Eastern, Southern, and Western Africa | Yes |
| Tick-borne encephalitis virus | Tick-borne encephalitis | 7–14 days | Fever, headache, muscle pain, nausea, vomiting, meningitis, and encephalitis | Paralysis and long-term brain damage | 1–2% | Ixodes scapularis, Ixodes ricinus, and Ixodes persulcatus | Small rodents | Eastern Europe and Southern Russia | Yes | |
| West Nile virus | West Nile fever, encephalitis | 2–15 days | Asymptomatic in most cases; fever, headache, fatigue, nausea, vomiting, rash | 3–6 days | Swollen lymph nodes, meningitis, encephalitis, acute flaccid paralysis | 3–15% in severe cases | Culex mosquitoes | Passerine birds | North America, Europe, West and Central Asia, Oceania, and Africa | Yes |
| Yellow fever virus | Yellow fever | 3–6 days | Fever, headache, back pain, loss of appetite, nausea, and vomiting | 3–4 days | Jaundice, liver damage, gastrointestinal bleeding, recurring fever | 3% in general; 20% in cases with severe complications | Aedes mosquitoes, especially Aedes aegypti | Primates | Tropical and subtropical regions of South America and Africa | Yes |
- Infection provides lifelong immunity to the specific serotype causing illness, but temporary immunity to other serotypes.
Cause
Transmission

Arboviruses maintain themselves in nature by going through a cycle between a host, an organism that carries the virus, and a vector, an organism that carries and transmits the virus to other organisms.[9] For arboviruses, vectors are commonly mosquitoes, ticks, sandflies[10] and other arthropods that consume the blood of vertebrates for nutritious or developmental purposes.[11] Vertebrates which have their blood consumed act as the hosts, with each vector generally having an affinity for the blood of specific species, making those species the hosts.[12]
Transmission between the vector and the host occurs when the vector feeds on the blood of the vertebrate, wherein the virus that has established an infection in the salivary glands of the vector comes into contact with the host's blood.[13][14] While the virus is inside the host, it undergoes a process called amplification, where the virus replicates at sufficient levels to induce viremia, a condition in which there are large numbers of viruses present in the blood.[15] The abundance of viruses in the host's blood allows the host to transmit the virus to other organisms if its blood is consumed by them. When uninfected vectors become infected from feeding, they are then capable of transmitting the virus to uninfected hosts, resuming amplification of virus populations. If viremia is not achieved in a vertebrate, the species can be called a "dead-end host", as the virus cannot be transmitted back to the vector.[16]
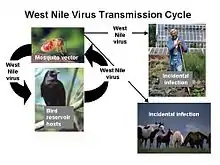
An example of this vector-host relationship can be observed in the transmission of the West Nile virus. Female mosquitoes of the genus Culex prefer to consume the blood of passerine birds, making them the hosts of the virus.[17] When these birds are infected, the virus amplifies, potentially infecting multiple mosquitoes that feed on its blood.[15] These infected mosquitoes may go on to further transmit the virus to more birds. If the mosquito is unable to find its preferred food source, it will choose another. Human blood is sometimes consumed, but since the West Nile virus does not replicate that well in mammals, humans are considered a dead-end host.[16][18]
In humans
Person-to-person transmission of arboviruses is not common, but can occur. Blood transfusions, organ transplantation, and the use of blood products can transmit arboviruses if the virus is present in the donor's blood or organs.[19][20][21] Because of this, blood and organs are often screened for viruses before being administered.[21][22] Rarely, vertical transmission, or mother-to-child transmission, has been observed in infected pregnant[23] and breastfeeding women.[24] Exposure to used needles may also transmit arboviruses if they have been used by an infected person or animal.[25] This puts intravenous drug users and healthcare workers at risk for infection in regions where the arbovirus may be spreading in human populations.[21][23]
Virology
Arboviruses are a polyphyletic group, belonging to various viral genera and therefore exhibiting different virologic characteristics.
| Arbovirus | Genome type | Genome length | Diameter | Capsid shape | Enveloped? | Viral entry | Replication site | Viral shedding | Infected cell(s) | Genetic variability |
|---|---|---|---|---|---|---|---|---|---|---|
| African swine fever virus | dsDNA | 170-190 kilobases | ~200 nm | Icosahedral | Yes | Endocytosis | Nucleus | Budding | Endothelial cells and red and white blood cells | 22 genotypes |
| Chikungunya virus (CHIKV) | +ssRNA | 11.6 kilobases | 60 - 70 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | Epithelial cells, endothelial cells, primary fibroblasts and macrophages | Three genotypes |
| Dengue virus | +ssRNA | ~11,000 nucleobases | ~50 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | Langerhans and white blood cells | Four serotypes |
| Japanese encephalitis virus | +ssRNA | ~11,000 nucleobases | ~50 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | Five genotypes | |
| Rift Valley fever virus | -ssRNA | Spherical | Yes | Cell cytoplasm | Budding | None[note 1] | ||||
| Tick-borne encephalitis virus | +ssRNA | ~11,000 nucleobases | 40-50 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | Neural cells | Five genotypes |
| West Nile virus | +ssRNA | ~11,000 nucleobases (11-12 kilo bases) | 45-50 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | ||
| Yellow fever virus | +ssRNA | ~11,000 nucleobases | 40-60 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding | Hepatocytes and white blood cells | |
| Zika virus | +ssRNA | 10794 nucleobases | 40 nm | Icosahedral | Yes | Membrane fusion | Cell cytoplasm | Budding |
- No significant distinct genetic populations exist due to the species having recent common ancestry.
Diagnosis
Preliminary diagnosis of arbovirus infection is usually based on clinical presentations of symptoms, places and dates of travel, activities, and epidemiological history of the location where infection occurred.[26] Definitive diagnosis is typically made in a laboratory by employing some combination of blood tests, particularly immunologic, serologic and/or virologic techniques such as ELISA,[26][27] complement fixation,[27] polymerase chain reaction,[27][28] neutralization test,[29] and hemagglutination-inhibition test.[30]
Classification
In the past, arboviruses were organized into one of four groups: A, B, C, and D. Group A denoted members of the genus Alphavirus,[31][32] Group B were members of the genus Flavivirus,[33] and Group C remains as the Group C serogroup of the genus Orthobunyavirus.[34] Group D was renamed in the mid-1950s to the Guama group and is currently the Guama serogroup in the genus Orthobunyavirus.[35] Currently, viruses are jointly classified according to Baltimore classification and a virus-specific system based on standard biological classification. With the exception of the African swine fever virus, which belongs to the Asfarviridae family of viruses, all major clinically important arboviruses belong to one of the following four groups:
- Order Bunyavirales (Baltimore class V)
- Genus Banyangvirus
- Genus Orthobunyavirus
- Bunyamwera virus
- California encephalitis virus
- Jamestown Canyon virus
- La Crosse encephalitis virus
- Genus Orthonairovirus
- Genus Phlebovirus
- Heartland virus
- Rift Valley fever virus
- Toscana virus
- Family Flaviviridae (Baltimore class IV)
- Genus Flavivirus
- Mosquito-borne viruses
- Dengue virus group
- Japanese encephalitis virus group
- Japanese encephalitis virus
- Murray Valley encephalitis virus
- St. Louis encephalitis virus
- West Nile virus
- Spondweni virus group
- Yellow fever virus group
- Yellow fever virus
- Tick-borne viruses
- Mammalian tick-borne virus group
- Kyasanur forest disease virus
- Tick-borne encephalitis virus
- Mammalian tick-borne virus group
- Mosquito-borne viruses
- Genus Flavivirus
- Family Reoviridae (Baltimore class III)
- Subfamily Sedoreovirinae
- Genus Orbivirus
- African horse sickness virus
- Bluetongue disease virus
- Epizootic hemorrhagic disease virus
- Equine encephalosis virus
- Genus Seadornavirus
- Genus Orbivirus
- Subfamily Spinareovirinae
- Genus Coltivirus
- Colorado tick fever virus
- Genus Coltivirus
- Subfamily Sedoreovirinae
- Family Togaviridae (Baltimore class IV)
- Genus Alphavirus
- Chikungunya virus'
- Eastern equine encephalitis virus
- Ross River virus
- Venezuelan equine encephalitis virus
- Western equine encephalitis virus'
- Genus Alphavirus
Prevention
Vector control measures, especially mosquito control, are essential to reducing the transmission of disease by arboviruses. Habitat control involves draining swamps and removal of other pools of stagnant water (such as old tires, large outdoor potted plants, empty cans, etc.) that often serve as breeding grounds for mosquitoes. Insecticides can be applied in rural and urban areas, inside houses and other buildings, or in outdoor environments. They are often quite effective for controlling arthropod populations, though use of some of these chemicals is controversial, and some organophosphates and organochlorides (such as DDT) have been banned in many countries. Infertile male mosquitoes have been introduced in some areas in order to reduce the breeding rate of relevant mosquito species. Larvicides are also used worldwide in mosquito abatement programs. Temefos is a common mosquito larvicide.[36]

People can also reduce the risk of getting bitten by arthropods by employing personal protective measures such as sleeping under mosquito nets, wearing protective clothing, applying insect repellents such as permethrin and DEET to clothing and exposed skin, and (where possible) avoiding areas known to harbor high arthropod populations. Arboviral encephalitis can be prevented in two major ways: personal protective measures and public health measures to reduce the population of infected mosquitoes. Personal measures include reducing time outdoors particularly in early evening hours, wearing long pants and long sleeved shirts and applying mosquito repellent to exposed skin areas. Public health measures often require spraying of insecticides to kill juvenile (larvae) and adult mosquitoes.[37]
Treatment
Because the arboviral encephalitides are viral diseases, antibiotics are not an effective form of treatment and no effective antiviral drugs have yet been discovered. Treatment is supportive, attempting to deal with problems such as swelling of the brain, loss of the automatic breathing activity of the brain and other treatable complications like bacterial pneumonia.[1]
Aspirin and ibuprofen should not be taken in cases of dengue fever as it could increase the risk of bleeding and cause Dengue Shock Syndrome.[47]
Epidemiology
Most arboviruses are located in tropical areas, however as a group they have a global distribution. The warm climate conditions found in tropical areas allows for year-round transmission by the arthropod vectors. Other important factors determining geographic distribution of arthropod vectors include rainfall, humidity, and vegetation.[48]
Mapping methods such as GIS and GPS have allowed for spatial and temporal analyses of arboviruses. Tagging cases or breeding sites geographically has allowed for deeper examination of vector transmission.[49]
To see the epidemiology of specific arboviruses, the following resources hold maps, fact sheets, and reports on arboviruses and arboviral epidemics.
| Resource | Description | Link |
|---|---|---|
| World Health Organization | The WHO compiles studies and maps of the distribution, risk factors, and prevention of specific viruses.
The WHO also hosts DengueNet, a database which can be queried about Dengue cases. |
http://www.who.int/en/ |
| CDC ArboNet Dynamic Map | This interactive map is created by USGS using data from the CDC ArboNET. It provides distribution maps of cases in humans and vectors in the United States. | https://web.archive.org/web/20161215234534/http://diseasemaps.usgs.gov/mapviewer/ |
| Center for Disease Control ArboCatalog | The ArboCatalog documents probable arboviruses recorded by the Center for Disease Control, and provides detailed information about the viruses. | https://wwwn.cdc.gov/Arbocat/Default.aspx |
History
| Year | Event |
|---|---|
| 1800s | Dengue fever epidemics occur globally |
| 1898–1914 | First large scale effort to prevent arbovirus infection takes place in Florida, Havana, and the Panama Canal Zone |
| 1901 | First arbovirus, the yellow fever virus, is discovered |
| 1906 | Dengue fever transmission is discovered |
| 1936 | Tick-borne encephalitis virus is discovered |
| 1937 | Yellow fever vaccine is invented |
| 1937 | West Nile virus is discovered |
| 1950s | Japanese encephalitis vaccines are invented |
| 1980s | Insecticide treated mosquito nets are developed |
| 1999 | West Nile virus reaches the Western Hemisphere |
| Late 1900s | Dengue fever spreads globally |
Arboviruses were not known to exist until the rise of modern medicine, with the germ theory and an understanding that viruses were distinct from other microorganisms. The connection between arthropods and disease was not postulated until 1881 when Cuban doctor and scientist Carlos Finlay proposed that yellow fever may be transmitted by mosquitoes instead of human contact,[50] a reality that was verified by Major Walter Reed in 1901.[51] The primary vector, Aedes aegypti, had spread globally from the 15th to the 19th centuries as a result of globalization and the slave trade.[52] This geographic spreading caused dengue fever epidemics throughout the 18th and 19th centuries,[53] and later, in 1906, transmission by the Aedes mosquitoes was confirmed, making yellow fever and dengue fever the first two diseases known to be caused by viruses.[54]
Thomas Milton Rivers published the first clear description of a virus as distinct from a bacterium in 1927.[55][56] The discovery of the West Nile virus came in 1937,[57] and has since been found in Culex populations[58] causing epidemics throughout Africa, the Middle East, and Europe. The virus was introduced into the Western Hemisphere in 1999, sparking a series of epidemics.[59] During the latter half of the 20th century, Dengue fever reemerged as a global disease, with the virus spreading geographically due to urbanization, population growth, increased international travel, and global warming,[60] and continues to cause at least 50 million infections per year, making Dengue fever the most common and clinically important arboviral disease.[61][62]
Yellow fever, alongside malaria, was a major obstacle in the construction of the Panama Canal. French supervision of the project in the 1880s was unsuccessful because of these diseases, forcing the abandonment of the project in 1889.[63] During the American effort to construct the canal in the early 1900s, William C. Gorgas, the Chief Sanitary Officer of Havana, was tasked with overseeing the health of the workers. He had past success in eradicating the disease in Florida and Havana by reducing mosquito populations through draining nearby pools of water, cutting grass, applying oil to the edges of ponds and swamps to kill larvae, and capturing adult mosquitoes that remained indoors during the daytime.[64] Joseph Augustin LePrince, the Chief Sanitary Inspector of the Canal Zone, invented the first commercial larvicide, a mixture of carbolic acid, resin, and caustic soda, to be used throughout the Canal Zone.[65] The combined implementation of these sanitation measures led to a dramatic decline in the number of workers dying and the eventual eradication of yellow fever in the Canal Zone as well as the containment of malaria during the 10-year construction period. Because of the success of these methods at preventing disease, they were adopted and improved upon in other regions of the world.[63][66]
See also
- List of diseases spread by invertebrates
- List of insect-borne diseases
- Mosquito-borne disease
- Robovirus
- Tibovirus
- Tick-borne disease
References
- "CDC Information on Arboviral Encephalitides". Archived from the original on January 27, 2007. Retrieved 2007-02-07.
- Hubálek, Z.; Rudolf, I. (2012). "Tick-borne viruses in Europe". Parasitology Research. 111 (1): 9–36. doi:10.1007/s00436-012-2910-1. PMID 22526290. S2CID 18713459.
- "Plant arboviruses: major threats to food security". Microbiology Society. Microbiology Society. Retrieved 20 May 2022.
- Stephen J. Schueler; John H. Beckett; D. Scott Gettings (2 April 2008). "Arbovirus Infection Symptoms". freeMD. Archived from the original on 8 September 2008. Retrieved 22 June 2013.
- Mostashari, F.; Bunning, M. L.; Kitsutani, P. T.; Singer, D. A.; Nash, D.; Cooper, M. J.; Katz, N.; Liljebjelke, K. A.; Biggerstaff, B. J.; Fine, A. D.; Layton, M. C.; Mullin, S. M.; Johnson, A. J.; Martin, D. A.; Hayes, E. B.; Campbell, G. L. (2001). "Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey". The Lancet. 358 (9278): 261–264. doi:10.1016/S0140-6736(01)05480-0. PMID 11498211. S2CID 13074756.
- Reiter, P. (2010). "Yellow Fever and Dengue: A threat to Europe?". Eurosurveillance. 15 (10): 19509. doi:10.2807/ese.15.10.19509-en. PMID 20403310.
- Davis, L. E.; Debiasi, R.; Goade, D. E.; Haaland, K. Y.; Harrington, J. A.; Harnar, J. B.; Pergam, S. A.; King, M. K.; Demasters, B. K.; Tyler, K. L. (2006). "West Nile virus neuroinvasive disease". Annals of Neurology. 60 (3): 286–300. doi:10.1002/ana.20959. PMID 16983682. S2CID 30778922.
- "Human blood contains the secret ingredient for mosquito eggs". May 4, 2011. Archived from the original on June 30, 2013. Retrieved 6 April 2013.
- Last, J., ed. (2001). A Dictionary of Epidemiology. New York: Oxford University Press. pp. 185–186. ISBN 978-0-19-514169-6. OCLC 207797812.
- Depaquit, J.; Grandadam, M.; Fouque, F.; Andry, P. E.; Peyrefitte, C. (2010). "Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: A review". Euro Surveillance. 15 (10): 19507. PMID 20403307.
- "Life cycle of Hard Ticks that Spread Disease". Centers for Disease Control and Prevention (CDC). 26 July 2012. Retrieved 26 June 2013.
- Kuno, G.; Chang, G. -J. J. (2005). "Biological Transmission of Arboviruses: Reexamination of and New Insights into Components, Mechanisms, and Unique Traits as Well as Their Evolutionary Trends". Clinical Microbiology Reviews. 18 (4): 608–637. doi:10.1128/CMR.18.4.608-637.2005. PMC 1265912. PMID 16223950.
- Wasserman, H. A.; Singh, S.; Champagne, D. E. (2004). "Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function". Parasite Immunology. 26 (6–7): 295–306. doi:10.1111/j.0141-9838.2004.00712.x. PMID 15541033. S2CID 32742815.
- Schneider, B. S.; McGee, C. E.; Jordan, J. M.; Stevenson, H. L.; Soong, L.; Higgs, S. (2007). Baylis, Matthew (ed.). "Prior Exposure to Uninfected Mosquitoes Enhances Mortality in Naturally-Transmitted West Nile Virus Infection". PLOS ONE. 2 (11): e1171. Bibcode:2007PLoSO...2.1171S. doi:10.1371/journal.pone.0001171. PMC 2048662. PMID 18000543.
- Weaver, S. C. (2005). "Host range, amplification and arboviral disease emergence". Archives of Virology. Supplementum (19): 33–44. doi:10.1007/3-211-29981-5_4. ISBN 3-211-24334-8. PMID 16358422.
- Bowen, R. A.; Nemeth, N. M. (2007). "Experimental infections with West Nile virus". Current Opinion in Infectious Diseases. 20 (3): 293–297. doi:10.1097/QCO.0b013e32816b5cad. PMID 17471040. S2CID 28937401.
- Lura, T.; Cummings, R.; Velten, R.; De Collibus, K.; Morgan, T.; Nguyen, K.; Gerry, A. (2012). "Host (avian) biting preference of southern California Culex mosquitoes (Diptera: Culicidae)". Journal of Medical Entomology. 49 (3): 687–696. doi:10.1603/ME11177. PMID 22679878. S2CID 20531226.
- Amraoui, F.; Krida, G.; Bouattour, A.; Rhim, A.; Daaboub, J.; Harrat, Z.; Boubidi, S. C.; Tijane, M.; Sarih, M.; Failloux, A. B. (2012). Ikegami, Tetsuro (ed.). "Culex pipiens, an Experimental Efficient Vector of West Nile and Rift Valley Fever Viruses in the Maghreb Region". PLOS ONE. 7 (5): e36757. Bibcode:2012PLoSO...736757A. doi:10.1371/journal.pone.0036757. PMC 3365064. PMID 22693557.
- Tambyah, P. A.; Koay, E. S. C.; Poon, M. L. M.; Lin, R. V. T. P.; Ong, B. K. C.; Transfusion-Transmitted Dengue Infection Study Group (2008). "Dengue Hemorrhagic Fever Transmitted by Blood Transfusion". New England Journal of Medicine. 359 (14): 1526–1527. doi:10.1056/NEJMc0708673. PMID 18832256.
- Iwamoto, M.; Jernigan, D. B.; Guasch, A.; Trepka, M. J.; Blackmore, C. G.; Hellinger, W. C.; Pham, S. M.; Zaki, S.; Lanciotti, R. S.; Lance-Parker, S. E.; Diazgranados, C. A.; Winquist, A. G.; Perlino, C. A.; Wiersma, S.; Hillyer, K. L.; Goodman, J. L.; Marfin, A. A.; Chamberland, M. E.; Petersen, L. R.; West Nile Virus in Transplant Recipients Investigation Team (2003). "Transmission of West Nile Virus from an Organ Donor to Four Transplant Recipients". New England Journal of Medicine. 348 (22): 2196–2203. doi:10.1056/NEJMoa022987. PMID 12773646. S2CID 19419227.
- Teo, D.; Ng, L. C.; Lam, S. (2009). "Is dengue a threat to the blood supply?". Transfusion Medicine. 19 (2): 66–77. doi:10.1111/j.1365-3148.2009.00916.x. PMC 2713854. PMID 19392949.
- Centers for Disease Control and Prevention (CDC) (2004). "Update: West Nile virus screening of blood donations and transfusion-associated transmission--United States, 2003". MMWR. Morbidity and Mortality Weekly Report. 53 (13): 281–284. PMID 15071426.
- Wiwanitkit, V. (2009). "Unusual mode of transmission of dengue". Journal of Infection in Developing Countries. 4 (1): 51–54. doi:10.3855/jidc.145. PMID 20130380.
- Centers for Disease Control and Prevention (CDC) (2002). "Possible West Nile virus transmission to an infant through breast-feeding--Michigan, 2002". MMWR. Morbidity and Mortality Weekly Report. 51 (39): 877–878. PMID 12375687.
- Venter, M.; Swanepoel, R. (2010). "West Nile Virus Lineage 2 as a Cause of Zoonotic Neurological Disease in Humans and Horses in Southern Africa". Vector-Borne and Zoonotic Diseases. 10 (7): 659–664. doi:10.1089/vbz.2009.0230. hdl:2263/16794. PMID 20854018. S2CID 25170132.
- "Arboviral Diagnostic Testing". Centers for Disease Control and Prevention (CDC). Retrieved April 17, 2013.
- "Arbovirus Antibodies Test". Medical Health Tests. March 27, 2012. Retrieved April 17, 2013.
- Huang, C.; Slater, B.; Campbell, W.; Howard, J.; White, D. (2001). "Detection of arboviral RNA directly from mosquito homogenates by reverse-transcription-polymerase chain reaction". Journal of Virological Methods. 94 (1–2): 121–128. doi:10.1016/s0166-0934(01)00279-8. PMID 11337046.
- Seawright, G. L.; Harding, G.; Thomas, F. C.; Hanson, R. P. (1974). "Microculture Plaque Neutralization Test for California Group Arboviruses". Applied Microbiology. 28 (5): 802–806. doi:10.1128/AEM.28.5.802-806.1974. PMC 186828. PMID 4216288.
- Mettler, N. E.; Clarke, D. H.; Casals, J. (1971). "Hemagglutination Inhibition with Arboviruses: Relationship Between Titers and Source of Erythrocytes". Applied Microbiology. 22 (3): 377–379. doi:10.1128/AEM.22.3.377-379.1971. PMC 376317. PMID 5165837.
- Dalrymple, J. M.; Vogel, S. N.; Teramoto, A. Y.; Russell, P. K. (1973). "Antigenic components of group a arbovirus virions". Journal of Virology. 12 (5): 1034–1042. doi:10.1128/JVI.12.5.1034-1042.1973. PMC 356734. PMID 4128825.
- Tesh, R. B.; Gajdusek, D. C.; Garruto, R. M.; Cross, J. H.; Rosen, L. (1975). "The distribution and prevalence of group a arbovirus neutralizing antibodies among human populations in Southeast Asia and the Pacific islands". The American Journal of Tropical Medicine and Hygiene. 24 (4): 664–675. doi:10.4269/ajtmh.1975.24.664. PMID 1155702.
- Lvov, D. K.; Tsyrkin, Y. M.; Karas, F. R.; Timopheev, E. M.; Gromashevski, V. L.; Veselovskaya, O. V.; Osipova, N. Z.; Fomina, K. B.; Grebenyuk, Y. I. (1973). "'Sokuluk' Virus, a new group B arbovirus isolated from Vespertilio pipistrellus Schreber, 1775, bat in the Kirghiz S.S.R". Archiv für die Gesamte Virusforschung. 41 (3): 170–174. doi:10.1007/BF01252762. PMID 4727779. S2CID 625707.
- Mezencio, J. M. S.; Peixoto, M. L. P.; Ferreira, P. C. P.; Golgher, R. R. (1978). "Induction of interferon by group C arboviruses". Archives of Virology. 58 (4): 355–358. doi:10.1007/BF01317828. PMID 104697. S2CID 39810753.
- Shope, R. E.; Woodall, J. P.; da Rosa, A. T. (1988). Monath, T. P. (ed.). The Arboviruses: Epidemiology and Ecology (PDF). Vol. 3. CRC Press. p. 38. ISBN 978-0849343872. Retrieved 16 June 2013.
- Walsh, J. A; Warren, K. S (1980). "Selective primary health care: An interim strategy for disease control in developing countries". Social Science & Medicine. Part C: Medical Economics. 14 (2): 145–63. doi:10.1016/0160-7995(80)90034-9. PMID 7403901.
- "Preventing Mosquito Bites". North Carolina Department of Health and Human Services.
- "Japanese Encephalitis Vaccine, What You Need to Know" (PDF). Centers for Disease Control and Prevention (CDC). December 7, 2011. Archived from the original (PDF) on 9 March 2013. Retrieved 20 March 2013.
- "Yellow Fever Vaccine, What You Need to Know" (PDF). Centers for Disease Control and Prevention (CDC). March 30, 2011. Retrieved 20 March 2013.
- "Tick-borne Encephalitis". World Health Organization (WHO). Archived from the original on October 4, 2014. Retrieved 5 November 2019.
- Ikegami, Tetsuro (2019). "Candidate vaccines for human Rift Valley fever". Expert Opin Biol Ther. 19 (Sep 3): 1333–1342. doi:10.1080/14712598.2019.1662784. PMID 31478397. S2CID 201805546.
- "Database Access - UNSW Library".
- "Dengue fever vaccine program". Global Vaccines. Archived from the original on 9 January 2013. Retrieved 20 March 2013.
- Pandya, Jyotsna; Gorchakov, Rodion; Wang, Eryu; Leal, Grace; Weaver, Scott C (2012). "A vaccine candidate for eastern equine encephalitis virus based on IRES-mediated attenuation". Vaccine. 30 (7): 1276–82. doi:10.1016/j.vaccine.2011.12.121. PMC 3283035. PMID 22222869.
- Young, S. (August 12, 2012). "Few Options in the West Nile Fight". MIT Technology Review. Retrieved 20 March 2013.
- Tharmarajah, Kothila; Mahalingam, Suresh; Zaid, Ali (2017). "Chikungunya: vaccines and therapeutics". F1000Research. 6 (Dec 8): 2114. doi:10.12688/f1000research.12461.1. PMC 5728195. PMID 29259782.
- "WHO | the human".
- Gubler, Duane J (2002). "The Global Emergence/Resurgence of Arboviral Diseases As Public Health Problems". Archives of Medical Research. 33 (4): 330–42. doi:10.1016/S0188-4409(02)00378-8. PMID 12234522.
- Petersen, L. R; Busch, M. P (2010). "Transfusion-transmitted arboviruses". Vox Sanguinis. 98 (4): 495–503. doi:10.1111/j.1423-0410.2009.01286.x. PMID 19951309. S2CID 29858335.
- Chaves-Carballo, E. (2005). "Carlos Finlay and yellow fever: Triumph over adversity". Military Medicine. 170 (10): 881–885. doi:10.7205/milmed.170.10.881. PMID 16435764.
- Russell, F. F. (1934). "Permanent Value of Major Walter Reed's Work on Yellow Fever *". American Journal of Public Health and the Nation's Health. 24 (1): 1–7. doi:10.2105/AJPH.24.1.1. PMC 1558495. PMID 18013904.
- Simmons, C. P.; Farrar, J. J.; Nguyen, N.; Wills, B. (2012). "Dengue". New England Journal of Medicine. 366 (15): 1423–1432. doi:10.1056/NEJMra1110265. hdl:11343/191104. PMID 22494122.
- Gubler, D. J. (1998). "Dengue and dengue hemorrhagic fever". Clinical Microbiology Reviews. 11 (3): 480–496. doi:10.1128/CMR.11.3.480. PMC 88892. PMID 9665979.
- Henchal, E. A.; Putnak, J. R. (1990). "The dengue viruses". Clinical Microbiology Reviews. 3 (4): 376–396. doi:10.1128/CMR.3.4.376. PMC 358169. PMID 2224837.
- Rivers, TM (October 1927). "Filterable Viruses a Critical Review". Journal of Bacteriology. 14 (4): 217–58. doi:10.1128/jb.14.4.217-258.1927. PMC 374955. PMID 16559270.
- Calisher, Charles H. (2013). Lifting the impenetrable veil : from yellow fever to Ebola hemorrhagic fever and SARS (1st ed.). Red Feather Lakes, Colo.: Rockpile Press. ISBN 978-0615827735.
- Smithburn, K. C.; Hughes, T. P.; Burke, A. W.; Paul, J. H. (1940). "A Neurotropic Virus Isolated from the Blood of a Native of Uganda". American Journal of Tropical Medicine and Hygiene. 20 (4): 471–472. doi:10.4269/ajtmh.1940.s1-20.471.
- Taylor, R. M.; Hurlbut, H. S.; Dressler, H. R.; Spangler, E. W.; Thrasher, D. (1953). "Isolation of West Nile virus from Culex mosquitoes". The Journal of the Egyptian Medical Association. 36 (3): 199–208. PMID 13084817.
- Sun, L. H. (13 September 2012). "West Nile epidemic on track to be deadliest ever: CDC". The Washington Post. Archived from the original on 24 June 2013. Retrieved 19 June 2013.
- Whitehorn, J.; Farrar, J. (2010). "Dengue". British Medical Bulletin. 95: 161–173. doi:10.1093/bmb/ldq019. PMID 20616106. S2CID 215154729.
- Rodenhuis-Zybert, I. A.; Wilschut, J.; Smit, J. M. (2010). "Dengue virus life cycle: Viral and host factors modulating infectivity". Cellular and Molecular Life Sciences. 67 (16): 2773–2786. doi:10.1007/s00018-010-0357-z. PMID 20372965. S2CID 4232236.
- Guzman, M. G.; Halstead, S. B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D. J.; Hunsperger, E.; Kroeger, A.; Margolis, H. S.; Martínez, E.; Nathan, M. B.; Pelegrino, J. L.; Simmons, C.; Yoksan, S.; Peeling, R. W. (2010). "Dengue: A continuing global threat". Nature Reviews Microbiology. 8 (12): S7–16. doi:10.1038/nrmicro2460. PMC 4333201. PMID 21079655.
- "Tropical Diseases and the Construction of the Panama Canal, 1904–1914". Contagion: Historical Views of Diseases and Epidemics. Retrieved 19 June 2013.
- "Malaria: The Panama Canal". Centers for Disease Control and Prevention (CDC). 8 February 2010. Retrieved 19 June 2013.
- Lapointe, P. M. (1987). "Joseph Augustin LePrince: His battle against mosquitoes and malaria". The West Tennessee Historical Society Papers. West Tennessee Historical Society. 41: 48–61. PMID 12862098.
- "Yellow Fever and Malaria in the Canal". PBS. American Experience. Retrieved 19 June 2013.
External links
- Beran, G. W., ed. (1994). Handbook of Zoonoses. CRC Press. ISBN 9780849332067.