Biguanide
Biguanide (/baɪˈɡwɒnaɪd/) is the organic compound with the formula HN(C(NH)NH2)2. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to ammonia and urea.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Imidodicarbonimidic diamide[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
507183 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.229 |
| EC Number |
|
Gmelin Reference |
240093 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C2H7N5 |
| Molar mass | 101.113 g·mol−1 |
| Acidity (pKa) | 3.07, 13.25 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Biguanide can be obtained from the reaction of dicyandiamide with ammonia, via a Pinner-type process.
Biguanide was first synthesized by Bernhard Rathke in 1879.[3]
Biguanidine drugs
A variety of derivatives of biguanide are used as pharmaceutical drugs.
Antihyperglycemic agents
The term "biguanidine" often refers specifically to a class of drugs that function as oral antihyperglycemic drugs used for diabetes mellitus or prediabetes treatment.[4]
Examples include:
- Metformin - widely used in treatment of diabetes mellitus type 2
- Phenformin - withdrawn from the market in most countries due to toxic effects
- Buformin - withdrawn from the market due to toxic effects
- bioactive biguanidines
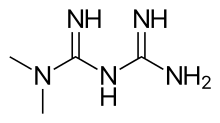 Metformin, an asymmetric dimethylbiguanidine
Metformin, an asymmetric dimethylbiguanidine Buformin. A butyl derivative of biguanidine.
Buformin. A butyl derivative of biguanidine.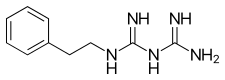 Phenformin. A phenethylated biguanidine.
Phenformin. A phenethylated biguanidine.
History
Galega officinalis (French lilac) was used in diabetes treatment for centuries.[5] In the 1920s, guanidine compounds were discovered in Galega extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, synthalin A and synthalin B, were used for diabetes treatment, but after the discovery of insulin, their use declined. Biguanides were reintroduced into Type 2 diabetes treatment in the late 1950s. Initially phenformin was widely used, but its potential for sometimes fatal lactic acidosis resulted in its withdrawal from most pharmacopeias (in the U.S. in 1978).[6] Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide.
Mechanism of action
The mechanism of action of biguanides is not fully understood, and many mechanisms have been proposed for metformin.
Biguanides do not affect the output of insulin, unlike other hypoglycemic agents such as sulfonylureas and meglitinides. Therefore, they are effective in Type 2 diabetics; and in Type 1 diabetes when used in conjunction with insulin therapy.
Mainly used in Type II diabetes, metformin is considered to increase insulin sensitivity in vivo, resulting in reduced plasma glucose concentrations, increased glucose uptake, and decreased gluconeogenesis.
However, in hyperinsulinemia, biguanides can lower fasting levels of insulin in plasma. Their therapeutic uses derive from their tendency to reduce gluconeogenesis in the liver, and, as a result, reduce the level of glucose in the blood. Biguanides also tend to make the cells of the body more willing to absorb glucose already present in the bloodstream, and there again reducing the level of glucose in the plasma.
Side effects and toxicity
The most common side effect is diarrhea and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is lactic acidosis, therefore metformin is contraindicated in advanced chronic kidney disease. Kidney function should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), hypoglycemia and other side effects are possible.
Antimalarial
Some biguanides are also used as antimalarial drugs. Examples include:
Disinfectants
The disinfectants chlorhexidine, polyaminopropyl biguanide (PAPB), polihexanide, and alexidine feature biguanide functional groups.[7]
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 885. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Güthner T, Mertschenk B, Schulz B (2006). "Guanidine and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_545.pub2. ISBN 3527306730.
- Rathke, B. (January 1879). "Ueber Biguanid". Berichte der deutschen chemischen Gesellschaft. 12 (1): 776–784. doi:10.1002/cber.187901201219.
- Rang HP, Dale MM, Ritter KM, Moore PK (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. p. 388. ISBN 0-443-07145-4.
- Witters L (2001). "The blooming of the French lilac". J Clin Invest. 108 (8): 1105–7. doi:10.1172/JCI14178. PMC 209536. PMID 11602616.
- Tonascia S, Meinert CL (1986). Clinical trials: design, conduct, and analysis. Oxford [Oxfordshire]: Oxford University Press. pp. 53–54, 59. ISBN 0-19-503568-2.
- Tanzer JM, Slee AM, Kamay BA (December 1977). "Structural requirements of guanide, biguanide, and bisbiguanide agents for antiplaque activity". Antimicrobial Agents and Chemotherapy. 12 (6): 721–9. doi:10.1128/aac.12.6.721. PMC 430011. PMID 931371.