Infigratinib
Infigratinib, sold under the brand name Truseltiq, is an anti-cancer medication used to treat cholangiocarcinoma (bile duct cancer).[1][4][5]
 | |
| Clinical data | |
|---|---|
| Trade names | Truseltiq |
| Other names | BGJ-398 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
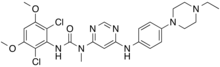
| Formula | C26H31Cl2N7O3 |
| Molar mass | 560.48 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Infigratinib is a kinase inhibitor targeting the fibroblast growth factor receptors FGFR1, FGFR2 and FGFR3[6][4][5] It was designated an orphan drug by the U.S. Food and Drug Administration (FDA) in 2019,[7] and it was approved for medical use in the United States in May 2021.[5]
Medical uses
Infigratinib is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma (bile duct cancer) with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.[4]
References
- "Truseltiq". Therapeutic Goods Administration (TGA). 22 November 2021. Retrieved 28 December 2021.
- "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Retrieved 13 May 2022.
- "Summary Basis of Decision (SBD) for Truseltiq". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- "Truseltiq- infigratinib capsule". DailyMed. Retrieved 10 June 2021.
- "BridgeBio Pharma's Affiliate QED Therapeutics and Partner Helsinn Group Announce FDA Approval of Truseltiq (infigratinib) for Patients with Cholangiocarcinoma" (Press release). BridgeBio Pharma. 28 May 2021. Retrieved 28 May 2021 – via GlobeNewswire.
- Botrus G, Raman P, Oliver T, Bekaii-Saab T (April 2021). "Infigratinib (BGJ398): an investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma". Expert Opinion on Investigational Drugs. 30 (4): 309–316. doi:10.1080/13543784.2021.1864320. PMID 33307867. S2CID 229177726.
- "Infigratinib Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 11 September 2019. Retrieved 30 May 2021.
External links
- "Infigratinib". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02150967 for "A Phase II, Single Arm Study of BGJ398 in Patients With Advanced Cholangiocarcinoma" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.