Trametinib
Trametinib (trade name Mekinist & Meqsel both by Novartis ) is a cancer drug. It is a MEK inhibitor drug with anti-cancer activity.[1]It inhibits MEK1 and MEK2.[1]
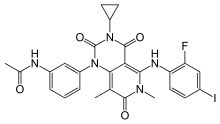 | |
| Clinical data | |
|---|---|
| Trade names | Mekinist |
| Other names | GSK1120212 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613040 |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.135 |
| Chemical and physical data | |
| Formula | C26H23FIN5O4 |
| Molar mass | 615.404 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Trametinib had good results for metastatic melanoma carrying the BRAF V600E mutation in a phase III clinical trial. In this mutation, the amino acid valine (V) at position 600 within the BRAF protein has become replaced by glutamic acid (E) making the mutant BRAF protein constitutively active.[2]
In May 2013, trametinib was approved as a single-agent by the Food and Drug Administration for the treatment of patients with V600E mutated metastatic melanoma.[3] Clinical trial data demonstrated that resistance to single-agent trametinib often occurs within 6 to 7 months.[4] To overcome this, trametinib was combined with the BRAF inhibitor dabrafenib.[4] As a result of this research, on January 8, 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of patients with BRAF V600E/K-mutant metastatic melanoma.[5] On May 1, 2018, the FDA approved the combination dabrafenib/trametinib as an adjuvant treatment for BRAF V600E-mutated, stage III melanoma after surgical resection based on the results of the COMBI-AD phase 3 study,[6] making it the first oral chemotherapy regimen that prevents cancer relapse for node positive, BRAF-mutated melanoma.[7]
Other uses
In a person with a sequence variant in the ARAF gene, trametinib helped the lymphatic system to remodel toward a healthier state, reducing lymphatic edema.[8] This benefit would not occur in most people because it is specific to genomes similar to the reported one, but it is potentially lifesaving for the few people with such genomes.[8] This case provides an example of what precision medicine can accomplish.[8]
References
- "Trametinib". NCI Drug Dictionary. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
- Robert C, Flaherty KT, Hersey P, Nathan PD, Garbe C, Milhem MM, et al. (31 January 2017). "METRIC phase III study: Efficacy of trametinib (T), a potent and selective MEK inhibitor (MEKi), in progression-free survival (PFS) and overall survival (OS), compared with chemotherapy (C) in patients (pts) with BRAFV600E/K mutant advanced or metastatic melanoma (MM)". Journal of Clinical Oncology. 30 (18_suppl): LBA8509. doi:10.1200/jco.2012.30.18_suppl.lba8509.
- "GSK melanoma drugs add to tally of U.S. drug approvals". Reuters. May 30, 2013.
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. (November 2012). "Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations". The New England Journal of Medicine. 367 (18): 1694–703. doi:10.1056/NEJMoa1210093. PMC 3549295. PMID 23020132.
- "Dabrafenib/Trametinib Combination Approved for Advanced Melanoma". OncLive. January 9, 2014.
- Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. (November 2017). "Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma" (PDF). The New England Journal of Medicine. 377 (19): 1813–1823. doi:10.1056/NEJMoa1708539. PMID 28891408. S2CID 205102412.
- "FDA Approves Adjuvant Combo for BRAF+ Melanoma". www.medscape.com. WebMD LLC. Retrieved 2 May 2018.
- Li, Dong; March, Michael E; Gutierrez-Uzquiza, Alvaro; Kao, Charlly; et al. (2019), "ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor", Nature Medicine, 25 (7): 1116–1122, doi:10.1038/s41591-019-0479-2, PMID 31263281, S2CID 195766663.