Transcription factor Jun
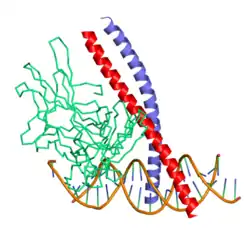
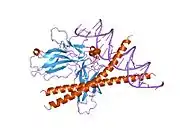
Transcription factor Jun is a protein that in humans is encoded by the JUN gene. c-Jun, in combination with protein c-Fos, forms the AP-1 early response transcription factor. It was first identified as the Fos-binding protein p39 and only later rediscovered as the product of the JUN gene. c-jun was the first oncogenic transcription factor discovered.[5] The proto-oncogene c-Jun is the cellular homolog of the viral oncoprotein v-jun (P05411).[6] The viral homolog v-jun was discovered in avian sarcoma virus 17 and was named for ju-nana, the Japanese word for 17.[7] The human JUN encodes a protein that is highly similar to the viral protein, which interacts directly with specific target DNA sequences to regulate gene expression. This gene is intronless and is mapped to 1p32-p31, a chromosomal region involved in both translocations and deletions in human malignancies.[8]
Function
Regulation
Both Jun and its dimerization partners in AP-1 formation are subject to regulation by diverse extracellular stimuli, which include peptide growth factors, pro-inflammatory cytokines, oxidative and other forms of cellular stress, and UV irradiation. For example, UV irradiation is a potent inducer for elevated c-jun expression.[6]
c-jun transcription is autoregulated by its own product, Jun. The binding of Jun (AP-1) to a high-affinity AP-1 binding site in the jun promoter region induces jun transcription. This positive autoregulation by stimulating its own transcription may be a mechanism for prolonging the signals from extracellular stimuli. This mechanism can have biological significance for the activity of c-jun in cancer.[9][10]
Also, the c-jun activities can be regulated by the ERK pathway. Constitutively active ERK is found to increase c-jun transcription and stability through CREB and GSK3. This results in activated c-jun and its downstream targets such as RACK1 and cyclin D1. RACK1 can enhance JNK activity, and activated JNK signaling subsequently exerts regulation on c-jun activity.[11]
It is activated through double phosphorylation by the JNK pathway but has also a phosphorylation-independent function. c-jun knockout is lethal, but transgenic animals with a mutated c-jun that cannot be phosphorylated (termed c-junAA) can survive.
Phosphorylation of Jun at serines 63 and 73 and threonine 91 and 93 increases transcription of the c-jun target genes.[12] Therefore, regulation of c-jun activity can be achieved through N-terminal phosphorylation by the Jun N-terminal kinases (JNKs). It is shown that Jun's activity (AP-1 activity) in stress-induced apoptosis and cellular proliferation is regulated by its N-terminal phosphorylation.[13] Another study showed that oncogenic transformation by ras and fos also requires Jun N-terminal phosphorylation at Serine 63 and 73.[14]
Cell cycle progression
Studies have shown that c-jun is required for progression through the G1 phase of the cell cycle, and c-jun null cells show increased G1 arrest. C-jun regulates the transcriptional level of cyclin D1, which is a major Rb kinase. Rb is a growth suppressor, and it is inactivated by phosphorylation. Therefore, c-jun is required for maintaining sufficient cyclin D1 kinase activity and allowing cell cycle progression.[6]
In cells absent of c-jun, the expression of p53 (cell cycle arrest inducer) and p21 (CDK inhibitor and p53 target gene) is increased, and those cells exhibit cell cycle defects. Overexpression of c-jun in cells results in decreased level of p53 and p21, and exhibits accelerated cell proliferation. C-jun represses p53 transcription by binding to a variant AP-1 site in the p53 promoter. Those results indicate that c-jun downregulates p53 to control cell cycle progression.[15]
Anti-apoptotic activity
UV irradiation can activate c-jun expression and the JNK signaling pathway. C-jun protects cells from UV-induced apoptosis, and it cooperates with NF-κB to prevent apoptosis induced by TNFα. The protection from apoptosis by c-jun requires serines 63/73 (involved in phosphorylation of Jun), which is not required in c-jun-mediated G1 progress. This suggests that c-jun regulates cell cycle progression and apoptosis through two separated mechanisms.[6]
A study utilized liver-specific inactivation of c-jun in hepatocellular carcinoma, which showed impaired tumor development correlated with increased level of p53 protein and the mRNA level of the p53 target gene noxa. Also, c-jun can protect hepatocytes from apoptosis, as hepatocytes lacking c-jun showed increased sensitivity to TNFα-induced apoptosis. In those hepatocytes lacking c-jun, deletion of p53 can restore resistance toward TNFα. Those results indicate that c-jun antagonizes the proapoptotic activity of p53 in liver tumor.[16]
Clinical significance
It is known that c-jun plays a role in cellular proliferation and apoptosis of the endometrium throughout the menstrual cycle. The cyclic change of the c-jun protein levels is significant in the proliferation and apoptosis of glandular epithelial cells. The persistent stromal expression of c-jun protein may prevent stromal cells from entering into apoptosis during the late secretory phase.[17]
Cancer
In a study using non-small cell lung cancers (NSCLC), c-jun was found to be overexpressed in 31% of the cases in primary and metastatic lung tumors, whereas normal conducting airway and alveolar epithelia in general did not express c-jun.[18]
A study with a group consisted of 103 cases of phase I/II invasive breast cancers showed that activated c-jun is expressed predominantly at the invasive front of breast cancer and is associated with proliferation and angiogenesis.[19]
Tumor initiation
A study was done with liver-specific inactivation of c-jun at different stages of tumor development in mice with chemically induced hepatocellular carcinomas. The result indicates that c-jun is required at the early stage of tumor development, and deletion of c-jun can largely suppress tumor formation. Also, c-jun is required for tumor cell survival between the initiation and progression stages. In contrast to that, inactivation of c-jun in advanced tumors does not impair tumor progression.[16]
Breast cancer
Overexpression of c-jun in MCF-7 cells can result in overall increased aggressiveness, as shown by increased cellular motility, increased expression of a matrix-degrading enzyme MMP-9, increased in vitro chemoinvasion, and tumor formation in nude mice in the absence of exogenous estrogens. The MCF-7 cells with c-jun overexpression became unresponsive to estrogen and tamoxifen, thus c-jun overexpression is proposed to lead to an estrogen-independent phenotype in breast cancer cells. The observed phenotype for MCF-7 cells with c-jun overexpression is similar to that observed clinically in advanced breast cancer, which had become hormone unresponsive.[20]
The invasive phenotype contributed by c-jun overexpression is confirmed in another study. In addition, this study showed increased in vivo liver metastasis by the breast cancer with c-jun overexpression. This finding suggests that c-jun plays a critical role in the metastasis of breast cancer.[21]
In mammary tumors, endogenous c-jun was found to play a key role in ErbB2-induced migration and invasion of mammary epithelial cells. Jun transcriptionally activates the promoters of SCF (stem cell factor) and CCL5. The induced SCF and CCL5 expression promotes a self-renewing mammary epithelial population. It suggests that c-jun mediates the expansion of breast cancer stem cells to enhance tumor invasiveness.[22]
Vulvar cancer
C-jun has been observed overexpressed in Vulvar Squamous Cell Carcinoma samples, in association with hypermethylation-Induced inactivation of the RARB tumor suppressor gene.[9] Indeed, mRNA levels of c-Jun tested higher in Vulvar cancer samples when compared with those of normal skin and preneoplastic vulvar lesions, thus underscoring a cross-link between RARB gene and the oncogene c-Jun.[9]
Cellular differentiation
Ten undifferentiated and highly aggressive sarcomas showed amplification of the jun gene and JUN overexpression at both RNA and protein levels. Overexpression of c-jun in 3T3-L1 cells (a preadipocytic non-tumoral cell line that resembles human liposarcoma) can block or delay adipocytic differentiation of those cells.[23]
Nerve and spinal cord regeneration
Peripheral nerve injury in rodents rapidly activates JNK signaling which in turn activates c-Jun. In contrast, nerve injury in the central nervous system does not. c-Jun is sufficient to promote axon regeneration in both the peripheral and central nervous systems as overexpression in both dorsal root ganglion neurons and cortical neurons leads to increased regeneration.[24]
As anti-cancer drug target
Since c-jun has been observed overexpressed in cancer,[9] several studies highlighted the hypothesis that this gene might be a target for cancer therapy. A study showed that oncogenic transformation by ras and fos requires Jun N-terminal phosphorylation at Serine 63 and 73 by the Jun N- terminal kinases (JNK). In this study, the induced skin tumor and osteosarcoma showed impaired development in mice with a mutant Jun incapable of N-terminal phosphorylation.[14] Also, in a mouse model of intestinal cancer, genetic abrogation of Jun N-terminal phosphorylation or gut-specific c-jun inactivation attenuated cancer development and prolonged lifespan.[12] Therefore, targeting the N-terminal phosphorylation of Jun (or the JNK signaling pathway) can be a potential strategy for inhibiting tumor growth.
In melanoma-derived B16-F10 cancer cells, c-jun inactivation by a pharmacological JNK/jun inhibitor SP combined with JunB knockdown can result in cytotoxic effect, leading to cell arrest and apoptosis. This anti-JunB /Jun strategy can increase the survival of mice inoculated with tumor cells, which suggests a potential antitumor strategy through Jun and JunB inhibition.[25]
Anti-cancer property of c-jun
Most research results show that c-jun contributes to tumor initiation and increased invasiveness. However, a few studies discovered some alternative activities of c-jun, suggesting that c-jun may actually be a double-edge sword in cancer.[26]
p16
p16INK4a is a tumor suppressor and a cell cycle inhibitor, and a study shows that c-jun acts as “bodyguard” to p16INK4a by preventing methylation of the p16INK4a promoter. Therefore, c-jun can prevent silencing of the gene p16INK4a.
Tylophorine
Tylophorine is a type of plant-derived alkaloid with anticancer activity by inducing cell cycle arrest. A study demonstrated that tylophorine treatment increased c-jun protein accumulation. Then c-jun expression in conjunction with tylophorine promotes G1 arrest in carcinoma cells through the downregulation of cyclin A2. Therefore, the result indicates that the anticancer mechanism of tylophorine is mediated through c-jun.[27]
Interactions
C-jun has been shown to interact with:
- ATF2[28][29][30]
- AR[31]
- ASCC3[32]
- ATF3[30][33][34]
- BCL3[35]
- BCL6[36]
- BRCA1[37]
- C-Fos[38][39][40][41][42][43][44]
- CSNK2A1[45]
- COPS5[46]
- CREBBP[47]
- CSNK2A2[45]
- DDX21,[48]
- DDIT3[49]
- ERG[50]
- ETS2,[51]
- FOSL1[39]
- GTF2B[52]
- MAPK8[53][54][55][56][57][58][59][60]
- MyoD[61]
- NACA[62]
- NELFB[63]
- NFE2L1[44]
- NFE2L2[44]
- NCOR2[64]
- NCOA1[65][66][67]
- PIN1[68]
- RBM39[69]
- RELA[41]
- RB1[70]
- RFWD2[71][72]
- RUNX1[73][74]
- RUNX2[73][74]
- SMAD3[75][76][77]
- STAT1[78]
- STAT3[78]
- TBP[52]
- TGIF1[79]
See also
References
- GRCh38: Ensembl release 89: ENSG00000177606 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000052684 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Vogt PK (June 2002). "Fortuitous convergences: the beginnings of JUN". Nature Reviews. Cancer. 2 (6): 465–9. doi:10.1038/nrc818. PMID 12189388. S2CID 44145552.
- Wisdom R, Johnson RS, Moore C (January 1999). "c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms". The EMBO Journal. 18 (1): 188–97. doi:10.1093/emboj/18.1.188. PMC 1171114. PMID 9878062.
- Maki Y, Bos TJ, Davis C, Starbuck M, Vogt PK (May 1987). "Avian sarcoma virus 17 carries the jun oncogene". Proceedings of the National Academy of Sciences of the United States of America. 84 (9): 2848–52. doi:10.1073/pnas.84.9.2848. PMC 304757. PMID 3033666.
- "Entrez Gene: JUN jun oncogene".
- Rotondo JC, Borghi A, Selvatici R, Mazzoni E, Bononi I, Corazza M, Kussini J, Montinari E, Gafà R, Tognon M, Martini F (July 2018). "Association of Retinoic Acid Receptor β Gene With Onset and Progression of Lichen Sclerosus-Associated Vulvar Squamous Cell Carcinoma". JAMA Dermatology. 154 (7): 819–823. doi:10.1001/jamadermatol.2018.1373. PMC 6128494. PMID 29898214.
- Angel P, Hattori K, Smeal T, Karin M (December 1988). "The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1". Cell. 55 (5): 875–85. doi:10.1016/0092-8674(88)90143-2. PMID 3142689. S2CID 19043736.
- Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S, Ronai Z (May 2007). "Rewired ERK-JNK signaling pathways in melanoma". Cancer Cell. 11 (5): 447–60. doi:10.1016/j.ccr.2007.03.009. PMC 1978100. PMID 17482134.
- Nateri AS, Spencer-Dene B, Behrens A (September 2005). "Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development". Nature. 437 (7056): 281–5. Bibcode:2005Natur.437..281N. doi:10.1038/nature03914. PMID 16007074. S2CID 4373376.
- Behrens A, Sibilia M, Wagner EF (March 1999). "Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation". Nature Genetics. 21 (3): 326–9. doi:10.1038/6854. PMID 10080190. S2CID 25622141.
- Behrens A, Jochum W, Sibilia M, Wagner EF (May 2000). "Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation". Oncogene. 19 (22): 2657–63. doi:10.1038/sj.onc.1203603. PMID 10851065.
- Schreiber M, Kolbus A, Piu F, Szabowski A, Möhle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF (March 1999). "Control of cell cycle progression by c-Jun is p53 dependent". Genes & Development. 13 (5): 607–19. doi:10.1101/gad.13.5.607. PMC 316508. PMID 10072388.
- Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF (January 2003). "Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53". Cell. 112 (2): 181–92. doi:10.1016/S0092-8674(03)00042-4. PMID 12553907. S2CID 8358992.
- Udou T, Hachisuga T, Tsujioka H, Kawarabayashi T (2004). "The role of c-jun protein in proliferation and apoptosis of the endometrium throughout the menstrual cycle". Gynecologic and Obstetric Investigation. 57 (3): 121–6. doi:10.1159/000075701. PMID 14691341. S2CID 29512406.
- Szabo E, Riffe ME, Steinberg SM, Birrer MJ, Linnoila RI (January 1996). "Altered cJUN expression: an early event in human lung carcinogenesis". Cancer Research. 56 (2): 305–15. PMID 8542585.
- Vleugel MM, Greijer AE, Bos R, van der Wall E, van Diest PJ (June 2006). "c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer". Human Pathology. 37 (6): 668–74. doi:10.1016/j.humpath.2006.01.022. PMID 16733206.
- Smith LM, Wise SC, Hendricks DT, Sabichi AL, Bos T, Reddy P, Brown PH, Birrer MJ (October 1999). "cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype". Oncogene. 18 (44): 6063–70. doi:10.1038/sj.onc.1202989. PMID 10557095.
- Zhang Y, Pu X, Shi M, Chen L, Song Y, Qian L, Yuan G, Zhang H, Yu M, Hu M, Shen B, Guo N (August 2007). "Critical role of c-Jun overexpression in liver metastasis of human breast cancer xenograft model". BMC Cancer. 7: 145. doi:10.1186/1471-2407-7-145. PMC 1959235. PMID 17672916.
- Jiao X, Katiyar S, Willmarth NE, Liu M, Ma X, Flomenberg N, Lisanti MP, Pestell RG (March 2010). "c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion". The Journal of Biological Chemistry. 285 (11): 8218–26. doi:10.1074/jbc.M110.100792. PMC 2832973. PMID 20053993.
- Mariani O, Brennetot C, Coindre JM, Gruel N, Ganem C, Delattre O, Stern MH, Aurias A (April 2007). "JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas". Cancer Cell. 11 (4): 361–74. doi:10.1016/j.ccr.2007.02.007. PMID 17418412.
- Mahar M, Cavalli V (June 2018). "Intrinsic mechanisms of neuronal axon regeneration". Nature Reviews. Neuroscience. 19 (6): 323–337. doi:10.1038/s41583-018-0001-8. PMC 5987780. PMID 29666508.
- Gurzov EN, Bakiri L, Alfaro JM, Wagner EF, Izquierdo M (January 2008). "Targeting c-Jun and JunB proteins as potential anticancer cell therapy". Oncogene. 27 (5): 641–52. doi:10.1038/sj.onc.1210690. PMID 17667939.
- Eferl, Robert; Wagner, Erwin F. (November 2003). "AP-1: a double-edged sword in tumorigenesis". Nature Reviews Cancer. 3 (11): 859–868. doi:10.1038/nrc1209. ISSN 1474-175X. PMID 14668816. S2CID 35328722.
- Yang CW, Lee YZ, Hsu HY, Wu CM, Chang HY, Chao YS, Lee SJ (June 2013). "c-Jun-mediated anticancer mechanisms of tylophorine". Carcinogenesis. 34 (6): 1304–14. doi:10.1093/carcin/bgt039. PMID 23385061.
- Newell CL, Deisseroth AB, Lopez-Berestein G (July 1994). "Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-alpha gene". Journal of Leukocyte Biology. 56 (1): 27–35. doi:10.1002/jlb.56.1.27. PMID 8027667. S2CID 85570533.
- Kara CJ, Liou HC, Ivashkiv LB, Glimcher LH (April 1990). "A cDNA for a human cyclic AMP response element-binding protein which is distinct from CREB and expressed preferentially in brain". Molecular and Cellular Biology. 10 (4): 1347–57. doi:10.1128/MCB.10.4.1347. PMC 362236. PMID 2320002.
- Hai T, Curran T (May 1991). "Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity". Proceedings of the National Academy of Sciences of the United States of America. 88 (9): 3720–4. Bibcode:1991PNAS...88.3720H. doi:10.1073/pnas.88.9.3720. PMC 51524. PMID 1827203.
- Sato N, Sadar MD, Bruchovsky N, Saatcioglu F, Rennie PS, Sato S, Lange PH, Gleave ME (July 1997). "Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP". The Journal of Biological Chemistry. 272 (28): 17485–94. doi:10.1074/jbc.272.28.17485. PMID 9211894.
- Jung DJ, Sung HS, Goo YW, Lee HM, Park OK, Jung SY, Lim J, Kim HJ, Lee SK, Kim TS, Lee JW, Lee YC (July 2002). "Novel transcription coactivator complex containing activating signal cointegrator 1". Molecular and Cellular Biology. 22 (14): 5203–11. doi:10.1128/MCB.22.14.5203-5211.2002. PMC 139772. PMID 12077347.
- Pearson AG, Gray CW, Pearson JF, Greenwood JM, During MJ, Dragunow M (December 2003). "ATF3 enhances c-Jun-mediated neurite sprouting". Brain Research. Molecular Brain Research. 120 (1): 38–45. doi:10.1016/j.molbrainres.2003.09.014. PMID 14667575.
- Chen BP, Wolfgang CD, Hai T (March 1996). "Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10". Molecular and Cellular Biology. 16 (3): 1157–68. doi:10.1128/MCB.16.3.1157. PMC 231098. PMID 8622660.
- Na SY, Choi JE, Kim HJ, Jhun BH, Lee YC, Lee JW (October 1999). "Bcl3, an IkappaB protein, stimulates activating protein-1 transactivation and cellular proliferation". The Journal of Biological Chemistry. 274 (40): 28491–6. doi:10.1074/jbc.274.40.28491. PMID 10497212.
- Vasanwala FH, Kusam S, Toney LM, Dent AL (August 2002). "Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene". Journal of Immunology. 169 (4): 1922–9. doi:10.4049/jimmunol.169.4.1922. PMID 12165517.
- Hu YF, Li R (June 2002). "JunB potentiates function of BRCA1 activation domain 1 (AD1) through a coiled-coil-mediated interaction". Genes & Development. 16 (12): 1509–17. doi:10.1101/gad.995502. PMC 186344. PMID 12080089.
- Ito T, Yamauchi M, Nishina M, Yamamichi N, Mizutani T, Ui M, Murakami M, Iba H (January 2001). "Identification of SWI.SNF complex subunit BAF60a as a determinant of the transactivation potential of Fos/Jun dimers". The Journal of Biological Chemistry. 276 (4): 2852–7. doi:10.1074/jbc.M009633200. PMID 11053448.
- Pognonec P, Boulukos KE, Aperlo C, Fujimoto M, Ariga H, Nomoto A, Kato H (May 1997). "Cross-family interaction between the bHLHZip USF and bZip Fra1 proteins results in down-regulation of AP1 activity". Oncogene. 14 (17): 2091–8. doi:10.1038/sj.onc.1201046. PMID 9160889.
- Glover JN, Harrison SC (January 1995). "Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA". Nature. 373 (6511): 257–61. Bibcode:1995Natur.373..257G. doi:10.1038/373257a0. PMID 7816143. S2CID 4276971.
- Yang X, Chen Y, Gabuzda D (September 1999). "ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB". The Journal of Biological Chemistry. 274 (39): 27981–8. doi:10.1074/jbc.274.39.27981. PMID 10488148.
- Nomura N, Zu YL, Maekawa T, Tabata S, Akiyama T, Ishii S (February 1993). "Isolation and characterization of a novel member of the gene family encoding the cAMP response element-binding protein CRE-BP1". The Journal of Biological Chemistry. 268 (6): 4259–66. doi:10.1016/S0021-9258(18)53604-8. PMID 8440710.
- Finkel T, Duc J, Fearon ER, Dang CV, Tomaselli GF (January 1993). "Detection and modulation in vivo of helix-loop-helix protein-protein interactions". The Journal of Biological Chemistry. 268 (1): 5–8. doi:10.1016/S0021-9258(18)54105-3. PMID 8380166.
- Venugopal R, Jaiswal AK (December 1998). "Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes". Oncogene. 17 (24): 3145–56. doi:10.1038/sj.onc.1202237. PMID 9872330.
- Yamaguchi Y, Wada T, Suzuki F, Takagi T, Hasegawa J, Handa H (August 1998). "Casein kinase II interacts with the bZIP domains of several transcription factors". Nucleic Acids Research. 26 (16): 3854–61. doi:10.1093/nar/26.16.3854. PMC 147779. PMID 9685505.
- Claret FX, Hibi M, Dhut S, Toda T, Karin M (October 1996). "A new group of conserved coactivators that increase the specificity of AP-1 transcription factors". Nature. 383 (6599): 453–7. Bibcode:1996Natur.383..453C. doi:10.1038/383453a0. PMID 8837781. S2CID 4353893.
- Sano Y, Tokitou F, Dai P, Maekawa T, Yamamoto T, Ishii S (October 1998). "CBP alleviates the intramolecular inhibition of ATF-2 function". The Journal of Biological Chemistry. 273 (44): 29098–105. doi:10.1074/jbc.273.44.29098. PMID 9786917.
- Westermarck J, Weiss C, Saffrich R, Kast J, Musti AM, Wessely M, Ansorge W, Séraphin B, Wilm M, Valdez BC, Bohmann D (February 2002). "The DEXD/H-box RNA helicase RHII/Gu is a co-factor for c-Jun-activated transcription". The EMBO Journal. 21 (3): 451–60. doi:10.1093/emboj/21.3.451. PMC 125820. PMID 11823437.
- Ubeda M, Vallejo M, Habener JF (November 1999). "CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins". Molecular and Cellular Biology. 19 (11): 7589–99. doi:10.1128/MCB.19.11.7589. PMC 84780. PMID 10523647.
- Verger A, Buisine E, Carrère S, Wintjens R, Flourens A, Coll J, Stéhelin D, Duterque-Coquillaud M (May 2001). "Identification of amino acid residues in the ETS transcription factor Erg that mediate Erg-Jun/Fos-DNA ternary complex formation" (PDF). The Journal of Biological Chemistry. 276 (20): 17181–9. doi:10.1074/jbc.M010208200. PMID 11278640. S2CID 32288807.
- Basuyaux JP, Ferreira E, Stéhelin D, Butticè G (October 1997). "The Ets transcription factors interact with each other and with the c-Fos/c-Jun complex via distinct protein domains in a DNA-dependent and -independent manner". The Journal of Biological Chemistry. 272 (42): 26188–95. doi:10.1074/jbc.272.42.26188. PMID 9334186.
- Franklin CC, McCulloch AV, Kraft AS (February 1995). "In vitro association between the Jun protein family and the general transcription factors, TBP and TFIIB". The Biochemical Journal. 305 (Pt 3): 967–74. doi:10.1042/bj3050967. PMC 1136352. PMID 7848298.
- Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K (December 2003). "Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling". The EMBO Journal. 22 (23): 6277–88. doi:10.1093/emboj/cdg605. PMC 291846. PMID 14633987.
- Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H (September 1998). "ASK1 is essential for JNK/SAPK activation by TRAF2". Molecular Cell. 2 (3): 389–95. doi:10.1016/S1097-2765(00)80283-X. PMID 9774977.
- Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (March 1994). "JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain". Cell. 76 (6): 1025–37. doi:10.1016/0092-8674(94)90380-8. PMID 8137421. S2CID 6797795.
- Yazgan O, Pfarr CM (August 2002). "Regulation of two JunD isoforms by Jun N-terminal kinases". The Journal of Biological Chemistry. 277 (33): 29710–8. doi:10.1074/jbc.M204552200. PMID 12052834.
- Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H (September 2001). "Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death". The Journal of Biological Chemistry. 276 (39): 36530–4. doi:10.1074/jbc.M104837200. PMID 11479302.
- Meyer CF, Wang X, Chang C, Templeton D, Tan TH (April 1996). "Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating kappaB enhancer activation". The Journal of Biological Chemistry. 271 (15): 8971–6. doi:10.1074/jbc.271.15.8971. PMID 8621542.
- Cano E, Hazzalin CA, Kardalinou E, Buckle RS, Mahadevan LC (November 1995). "Neither ERK nor JNK/SAPK MAP kinase subtypes are essential for histone H3/HMG-14 phosphorylation or c-fos and c-jun induction". Journal of Cell Science. 108 (Pt 11): 3599–609. doi:10.1242/jcs.108.11.3599. PMID 8586671.
- Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ (July 1997). "Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase". Proceedings of the National Academy of Sciences of the United States of America. 94 (14): 7337–42. Bibcode:1997PNAS...94.7337T. doi:10.1073/pnas.94.14.7337. PMC 23822. PMID 9207092.
- Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM (February 1992). "Functional antagonism between c-Jun and MyoD proteins: a direct physical association". Cell. 68 (3): 507–19. doi:10.1016/0092-8674(92)90187-H. PMID 1310896. S2CID 44966899.
- Moreau A, Yotov WV, Glorieux FH, St-Arnaud R (March 1998). "Bone-specific expression of the alpha chain of the nascent polypeptide-associated complex, a coactivator potentiating c-Jun-mediated transcription". Molecular and Cellular Biology. 18 (3): 1312–21. doi:10.1128/MCB.18.3.1312. PMC 108844. PMID 9488446.
- Zhong H, Zhu J, Zhang H, Ding L, Sun Y, Huang C, Ye Q (December 2004). "COBRA1 inhibits AP-1 transcriptional activity in transfected cells". Biochemical and Biophysical Research Communications. 325 (2): 568–73. doi:10.1016/j.bbrc.2004.10.079. PMID 15530430.
- Lee SK, Kim JH, Lee YC, Cheong J, Lee JW (April 2000). "Silencing mediator of retinoic acid and thyroid hormone receptors, as a novel transcriptional corepressor molecule of activating protein-1, nuclear factor-kappaB, and serum response factor". The Journal of Biological Chemistry. 275 (17): 12470–4. doi:10.1074/jbc.275.17.12470. PMID 10777532.
- Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW (November 1999). "A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo". The Journal of Biological Chemistry. 274 (48): 34283–93. doi:10.1074/jbc.274.48.34283. PMID 10567404.
- Lee SK, Na SY, Jung SY, Choi JE, Jhun BH, Cheong J, Meltzer PS, Lee YC, Lee JW (June 2000). "Activating protein-1, nuclear factor-kappaB, and serum response factor as novel target molecules of the cancer-amplified transcription coactivator ASC-2". Molecular Endocrinology. 14 (6): 915–25. doi:10.1210/mend.14.6.0471. PMID 10847592.
- Lee SK, Kim HJ, Na SY, Kim TS, Choi HS, Im SY, Lee JW (July 1998). "Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits". The Journal of Biological Chemistry. 273 (27): 16651–4. doi:10.1074/jbc.273.27.16651. PMID 9642216.
- Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, Lu KP (July 2001). "Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1". The EMBO Journal. 20 (13): 3459–72. doi:10.1093/emboj/20.13.3459. PMC 125530. PMID 11432833.
- Jung DJ, Na SY, Na DS, Lee JW (January 2002). "Molecular cloning and characterization of CAPER, a novel coactivator of activating protein-1 and estrogen receptors". The Journal of Biological Chemistry. 277 (2): 1229–34. doi:10.1074/jbc.M110417200. PMID 11704680.
- Nishitani J, Nishinaka T, Cheng CH, Rong W, Yokoyama KK, Chiu R (February 1999). "Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site". The Journal of Biological Chemistry. 274 (9): 5454–61. doi:10.1074/jbc.274.9.5454. PMID 10026157.
- Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, Dixit VM (February 2004). "Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase" (PDF). Science. 303 (5662): 1371–4. Bibcode:2004Sci...303.1371W. doi:10.1126/science.1093549. PMID 14739464. S2CID 40501515.
- Bianchi E, Denti S, Catena R, Rossetti G, Polo S, Gasparian S, Putignano S, Rogge L, Pardi R (May 2003). "Characterization of human constitutive photomorphogenesis protein 1, a RING finger ubiquitin ligase that interacts with Jun transcription factors and modulates their transcriptional activity". The Journal of Biological Chemistry. 278 (22): 19682–90. doi:10.1074/jbc.M212681200. PMID 12615916.
- Hess J, Porte D, Munz C, Angel P (June 2001). "AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element". The Journal of Biological Chemistry. 276 (23): 20029–38. doi:10.1074/jbc.M010601200. PMID 11274169.
- D'Alonzo RC, Selvamurugan N, Karsenty G, Partridge NC (January 2002). "Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation". The Journal of Biological Chemistry. 277 (1): 816–22. doi:10.1074/jbc.M107082200. PMID 11641401.
- Zhang Y, Feng XH, Derynck R (August 1998). "Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription". Nature. 394 (6696): 909–13. Bibcode:1998Natur.394..909Z. doi:10.1038/29814. PMID 9732876. S2CID 4393852.
- Verrecchia F, Pessah M, Atfi A, Mauviel A (September 2000). "Tumor necrosis factor-alpha inhibits transforming growth factor-beta /Smad signaling in human dermal fibroblasts via AP-1 activation". The Journal of Biological Chemistry. 275 (39): 30226–31. doi:10.1074/jbc.M005310200. PMID 10903323.
- Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang XF (April 1999). "Smads bind directly to the Jun family of AP-1 transcription factors". Proceedings of the National Academy of Sciences of the United States of America. 96 (9): 4844–9. Bibcode:1999PNAS...96.4844L. doi:10.1073/pnas.96.9.4844. PMC 21779. PMID 10220381.
- Zhang X, Wrzeszczynska MH, Horvath CM, Darnell JE (October 1999). "Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation". Molecular and Cellular Biology. 19 (10): 7138–46. doi:10.1128/MCB.19.10.7138. PMC 84707. PMID 10490649.
- Pessah M, Prunier C, Marais J, Ferrand N, Mazars A, Lallemand F, Gauthier JM, Atfi A (May 2001). "c-Jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity". Proceedings of the National Academy of Sciences of the United States of America. 98 (11): 6198–203. Bibcode:2001PNAS...98.6198P. doi:10.1073/pnas.101579798. PMC 33445. PMID 11371641.
Further reading
- Bohmann D, Bos TJ, Admon A, Nishimura T, Vogt PK, Tjian R (December 1987). "Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1". Science. 238 (4832): 1386–92. Bibcode:1987Sci...238.1386B. doi:10.1126/science.2825349. PMID 2825349.
- Rahmsdorf HJ (December 1996). "Jun: transcription factor and oncoprotein". Journal of Molecular Medicine. 74 (12): 725–47. doi:10.1007/s001090050077. PMID 8974016. S2CID 2693522.
- Liu JL, Kung HJ (2001). "Marek's disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style". Virus Genes. 21 (1–2): 51–64. doi:10.1023/A:1008132313289. PMID 11022789. S2CID 2303249.
- Velazquez Torres A, Gariglio Vidal P (2002). "[Possible role of transcription factor AP1 in the tissue-specific regulation of human papillomavirus]". Revista de Investigacion Clinica. 54 (3): 231–42. PMID 12183893.
- Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG (February 2007). "The activator protein-1 transcription factor in respiratory epithelium carcinogenesis". Molecular Cancer Research. 5 (2): 109–20. doi:10.1158/1541-7786.MCR-06-0311. PMID 17314269.
External links
- c-jun+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- c-jun+Genes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Drosophila Jun-related antigen - The Interactive Fly
- FactorBook C-Jun
- Human JUN genome location and JUN gene details page in the UCSC Genome Browser.