Aceperone
Aceperone is a neuroleptic drug of the butyrophenone class. It is an α-noradrenergic blocking drug developed by Janssen Pharmaceutica in the 1960s.[1]
 | |
| Clinical data | |
|---|---|
| Other names | Acetabuton; R 3248 |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
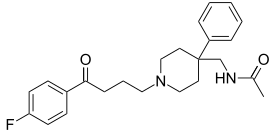
| Formula | C24H29FN2O2 |
| Molar mass | 396.506 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 97 to 100 °C (207 to 212 °F) [1] |
SMILES
| |
InChI
| |
Aceperone has been used as a tool in the study of the biochemical basis of learning. Although aceperone does not block learning per se, it blocks access to an attentional mechanism by which animals ‘tune in’ to the relevant visual dimension when learning a visual discrimination task[2][3] at doses below those that affect general behaviour.[4]
Synthesis
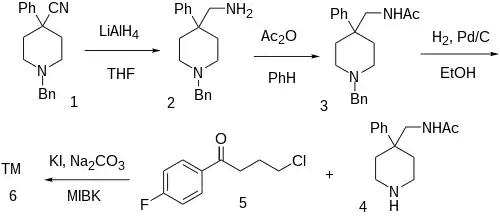
The reduction of 1-Benzyl-4-cyano-4-phenylpiperidine [56243-25-5] (1) with lithium aluminium hydride gives 1-Benzyl-4-phenylpiperidine-4-methylamine [84176-77-2] (2). Acetylation of this primary amine would yield [7152-05-8] (3). The removal of the benzyl protecting group by catalytic hydrogenation gives N-[(4-phenyl-4-piperidinyl)methyl]acetamide [83763-23-9] (4). Alkylation with 4-Chloro-4'-Fluorobutyrophenone [3874-54-2] (5) affords aceperone (6).
References
- BE 606849, Janssen PA, "Alkoxylamino and alkoxycarbonylamino derivatives of 1(aroylalkyl)-4-arylpiperidines.", published 1961
- Ridley RM, Haystead TA, Baker HF, Crow TJ (June 1981). "A new approach to the role of noradrenaline in learning: problem-solving in the marmoset after alpha-noradrenergic receptor blockade". Pharmacology, Biochemistry, and Behavior. 14 (6): 849–55. doi:10.1016/0091-3057(81)90373-7. PMID 6114497. S2CID 35341802.
- Baker HF, Ridley RM, Haystead TA, Crow TJ (May 1983). "Further consideration of the learning impairment after aceperone in the marmoset: effects of the drug on shape and colour discrimination and on an alternation task". Pharmacology, Biochemistry, and Behavior. 18 (5): 701–4. doi:10.1016/0091-3057(83)90009-6. PMID 6222386. S2CID 7905046.
- Scraggs PR, Ridley RM (March 1979). "The effect of dopamine and noradrenaline blockade on amphetamine-induced behaviour in the marmoset". Psychopharmacology. 62 (1): 41–5. doi:10.1007/BF00426033. PMID 155838. S2CID 38197514.
- BE606849 idem Paul A J Janssen, to U.S. Patent 3,083,205 (1963 to Research Laboratorium C Janssen NV).