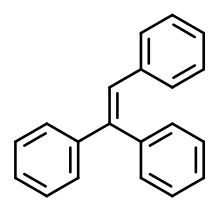
Triphenylethylene
Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity.[1][2] Its estrogenic effects were discovered in 1937.[3] TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.[4]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.359 |
| Chemical and physical data | |
| Formula | C20H16 |
| Molar mass | 256.348 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
TPE is the parent compound of a group of nonsteroidal estrogen receptor ligands.[1][2][5] It includes the estrogens chlorotrianisene, desmethylchlorotrianisene, estrobin (DBE), M2613, triphenylbromoethylene, triphenylchloroethylene, triphenyliodoethylene, triphenylmethylethylene; the selective estrogen receptor modulators (SERMs) afimoxifene, brilanestrant, broparestrol, clomifene, clomifenoxide, droloxifene, endoxifen, etacstil, fispemifene, idoxifene, miproxifene, miproxifene phosphate, nafoxidine, ospemifene, panomifene, and toremifene. The antiestrogen ethamoxytriphetol (MER-25) is also closely related, but is technically not a derivative of TPE and is instead a triphenylethanol derivative. The tamoxifen metabolite and aromatase inhibitor norendoxifen is also a TPE derivative. In addition to their estrogenic activity, various TPE derivatives like tamoxifen and clomifene have been found to act as protein kinase C inhibitors.[6]
The affinity of triphenylethylene for the rat estrogen receptor is about 0.002% relative to estradiol.[7][8] For comparison, the relative binding affinities of derivatives of triphenylethylene were 1.6% for tamoxifen, 175% for afimoxifene (4-hydroxytamoxifen), 15% for droloxifene, 1.4% for toremifene (4-chlorotamoxifen), 0.72% for clomifene, and 0.72% for nafoxidine.[9][7][8]
See also
- List of SERMs
- Benzothiophene – parent compound for another group of nonsteroidal SERMs that includes raloxifene
- Phenanthrene – parent compound of steroidal estrogens like estradiol
- Chrysene – parent compound of a group of nonsteroidal weak estrogens that includes 2,8-DHHHC and tetrahydrochrysene
- Doisynolic acid – parent compound of a group of nonsteroidal estrogens that includes doisynoestrol
- Allenolic acid – parent compound of a group of nonsteroidal estrogens that includes methallenestril
References
- JORDAN V. CRAIG; B.J.A. Furr (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 95–. ISBN 978-1-59259-152-7.
- Philipp Y. Maximov; Russell E. McDaniel; V. Craig Jordan (23 July 2013). Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. pp. 4–. ISBN 978-3-0348-0664-0.
- Jie Jack Li (3 April 2009). Triumph of the Heart: The Story of Statins. Oxford University Press, USA. pp. 33–. ISBN 978-0-19-532357-3.
- Carmen Avendano; J. Carlos Menendez (11 June 2015). Medicinal Chemistry of Anticancer Drugs. Elsevier Science. pp. 87–. ISBN 978-0-444-62667-7.
- Antonio Cano; Joacquim Calaf i Alsina; Jose Luis Duenas-Diez (22 September 2006). Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs. Springer Science & Business Media. pp. 52–. ISBN 978-3-540-34742-2.
- O'Brian CA, Liskamp RM, Solomon DH, Weinstein IB (1986). "Triphenylethylenes: a new class of protein kinase C inhibitors". J. Natl. Cancer Inst. 76 (6): 1243–6. doi:10.1093/jnci/76.6.1243. PMID 3458960.
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM (March 2000). "The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands". Toxicol Sci. 54 (1): 138–53. doi:10.1093/toxsci/54.1.138. PMID 10746941.
- Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL, Sheehan DM (March 2001). "Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens". Chem Res Toxicol. 14 (3): 280–94. doi:10.1021/tx000208y. PMID 11258977.
- Wittliff, J. L., Kerr II, D. A., & Andres, S. A. (2005). "Estrogens IV: Estrogen-Like Pharmaceuticals". In Wexler, P. (ed.). Encyclopedia of Toxicology, 2nd Edition. Vol. Dib–L. pp. 254–258. ISBN 9780080548005.
{{cite book}}: CS1 maint: uses authors parameter (link)