Ardeparin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| Routes of administration | Injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.590 |
| Chemical and physical data | |
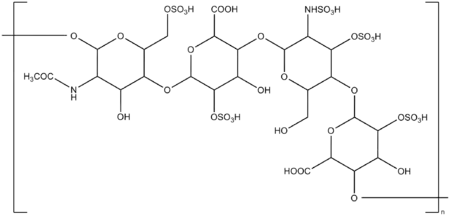
| Formula | (C26H40N2O36S5)n |
| Molar mass | 5500–6500 g/mol (average) |
| | |
Ardeparin (trade name Normiflo) is an anticoagulant. It was used for the prevention of deep vein thrombosis, but was withdrawn from the US market in 2000 for reasons unrelated to safety or efficacy.[1][2][3]
References
- ↑ Frishman WH, Cheng-Lai A, Chen J (2000). "Antithrombotic therapy". Current cardiovascular drugs (3rd ed.). Philadelphia, Pa.: Current Medicine. p. 90. ISBN 978-1-4615-6767-7.
- ↑ "Normiflo". Drugs.com. Archived from the original on 2017-02-19. Retrieved 2018-01-23.
- ↑ "Determination That Ardeparin Sodium Injection Was Not Withdrawn From Sale for Reasons of Safety or Effectiveness". United States Food and Drug Administration. Federal Register. Retrieved 24 June 2022.
External links
- "Heparin sodium". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.