Ciclosporin
 | |
| Names | |
|---|---|
| Pronunciation | /ˌsaɪkləˈspɔːrɪn/[1] |
| Trade names | Neoral, Sandimmune, others |
| Other names | cyclosporin, ciclosporin A,[2] cyclosporine A, cyclosporin A (CsA), cyclosporine (USAN US) |
IUPAC name
| |
| Clinical data | |
| Drug class | Immunosuppressant (calcineurin inhibitor) |
| Main uses | Rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, organ transplant[3][4] |
| Side effects | High blood pressure, headache, kidney problems, increased hair growth, vomiting[4] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous (IV), eye drops |
| External links | |
| AHFS/Drugs.com | Systemic Monograph Eye: Monograph |
| MedlinePlus | a601207 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Variable |
| Metabolism | liver CYP3A4 |
| Elimination half-life | Variable (about 24 hours) |
| Excretion | biliary |
| Chemical and physical data | |
| Formula | C62H111N11O12 |
| Molar mass | 1202.635 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ciclosporin, also spelled cyclosporine and cyclosporin, is an immunosuppressant medication and natural product.[3] It is taken by mouth or by injection into a vein for rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, and in organ transplants to prevent rejection.[3][4] It is also used as eye drops for keratoconjunctivitis sicca (dry eyes).[5]
Common side effects include high blood pressure, headache, kidney problems, increased hair growth, and vomiting.[4] Other severe side effects include an increased risk of infection, liver problems, and an increased risk of lymphoma.[4] Blood levels of the medication should be checked to decrease the risk of side effects.[4] Use during pregnancy may result in preterm birth; however, ciclosporin does not appear to cause birth defects.[6]
Ciclosporin is believed to work by decreasing the function of lymphocytes.[4] It does this by forming a complex with cyclophilin to block the phosphatase activity of calcineurin, which in turn decreases the production of inflammatory cytokines by T‐lymphocytes.[7]
Ciclosporin was isolated in 1971 from the fungus Tolypocladium inflatum and came into medical use in 1983.[8] It is on the World Health Organization's List of Essential Medicines.[9] In 2016, the wholesale cost in the developing world was about US$106 a month.[10] In the United Kingdom, it cost the NHS about £121 per month.[11] The wholesale price in the United States was about US$173 per month.[12] In 2017, it was the 248th most commonly prescribed medication in the United States, with more than one million prescriptions that year.[13][14]
Medical uses
Ciclosporin is approved by the FDA to treat and prevent graft-versus-host disease in bone marrow transplantation and to prevent rejection of kidney, heart, and liver transplants.[15][16] It is also approved in the US for treating of rheumatoid arthritis and psoriasis, persistent nummular keratitis following adenoviral keratoconjunctivitis,[17][16] and as eye drops for treating dry eyes caused by Sjögren's syndrome and meibomian gland dysfunction.[18]
In addition to these indications, ciclosporin is also used in severe atopic dermatitis, Kimura disease, pyoderma gangrenosum, chronic hives, acute systemic mastocytosis, and posterior or intermediate uveitis with noninfective cause. It is also used, albeit infrequently, in severe rheumatoid arthritis and related diseases.
Ciclosporin has also been used in people with acute severe ulcerative colitis and hives that do not respond to treatment with steroids.[19]
Side effects
.jpg.webp)
Side effects of ciclosporin can include gum enlargement, increased hair growth, convulsions, peptic ulcers, pancreatitis, fever, vomiting, diarrhea, confusion, increased cholesterol, trouble breathing, numbness and tingling (particularly of the lips), itchiness, high blood pressure, potassium retention (possibly leading to hyperkalemia), kidney and liver dysfunction,[20] burning sensations at finger tips, and an increased vulnerability to opportunistic fungal and viral infections. Ciclosporin causes hypertension by inducing vasoconstriction in the kidneys and increasing sodium reabsorption. The increase in blood pressure can cause cardiovascular events; it is thus recommended that the lowest effective dose for people requiring long-term treatment be used.[21]
Ciclosporin use after a kidney transplantation is associated with increased levels of uric acid in the blood and, in some cases, gout.[22] This is due to the decrease in glomerular filtration rate, which leads to uric acid retention. Use of azathioprine as an alternative has shown to reduce the incidence of gouty arthritis.
Ciclosporin is listed as an IARC Group 1 carcinogen (i.e. there is sufficient evidence of carcinogenicity in humans),[23] specifically leading to squamous cell skin cancer and non-Hodgkin lymphoma.[24]
Pharmacology
Mechanism of action
| Ciclosporin | |
|---|---|
| Identifiers | |
| Symbol | N/A |
| OPM superfamily | 94 |
| OPM protein | 1cwa |
Ciclosporin's main effect is to lower the activity of T-cells; it does so by inhibiting calcineurin in the calcineurin–phosphatase pathway and preventing the mitochondrial permeability transition pore from opening. Ciclosporin binds to the cytosolic protein cyclophilin (immunophilin) of lymphocytes, especially of T cells. This cyclosporin—cyclophilin complex inhibits calcineurin, which is normally responsible for activating the transcription of interleukin 2. In T-cells, activation of the T-cell receptor normally increases intracellular calcium, which acts via calmodulin to activate calcineurin. Calcineurin then dephosphorylates the transcription factor NF-AT (nuclear factor of activated T-cells), which moves to the T-cell nucleus and increases the transcription of genes for IL-2 and related cytokines.[7] Ciclosporin, by preventing the dephosphorylation of NF-AT, leads to reduced effector T-cell function;[25][26][27][28] it does not affect cytostatic activity.
Ciclosporin also binds to the cyclophilin D protein that constitutes part of the mitochondrial permeability transition pore (MPTP).[26][29] The MPTP is found in the mitochondrial membrane of cardiac muscle cells and moves calcium ions (Ca2+
) into the mitochondria.[26][29] When open, Ca2+
enters the mitochondria and causes the muscle cells (and thus the heart) to contract. If unregulated, the influx of Ca2+
can contribute to mitochondrial swelling and dysfunction.[29]
Pharmacokinetics
Ciclosporin is a cyclic peptide of 11 amino acids; it contains a single D-amino acid, which is rarely encountered in nature. Unlike most peptides, ciclosporin is not synthesized by ribosomes.[30]
Ciclosporin is highly metabolized in humans and animals after ingestion. The metabolites, which include cyclosporin B, C, D, E, H, and L,[31] have less than 10% of ciclosporin's immunosuppressant activity and are associated with higher kidney toxicity.[32] Individual ciclosporin metabolites have been isolated and characterized but do not appear to be extensively studied.
Biosynthesis
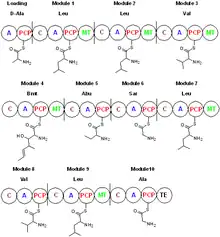
Cyclosporin is synthesized by a nonribosomal peptide synthetase, cyclosporin synthetase.[33] The enzyme contains an adenylation domain, a thiolation domain, a condensation domain, and an N-methyltransferase domain. The adenylation domain is responsible for substrate recognition and activation, whereas the thiolation domain covalently binds the adenylated amino acids to phosphopantetheine, and the condensation domain elongates the peptide chain. Cyclosporin synthetase substrates include L-valine, L-leucine, L-alanine, glycine, 2-aminobutyric acid, 4-methylthreonine, and D-alanine, which is the starting amino acid in the biosynthetic process.[34] With the adenylation domain, cyclosporin synthetase generates the acyl-adenylated amino acids, then covalently binds the amino acid to phosphopantetheine through a thioester linkage. Some of the amino acid substrates become N-methylated by S-adenosyl methionine. The cyclization step releases cyclosporin from the enzyme.[35] Amino acids such as D-Ala and butenyl-methyl-L-threonine (Bmt) indicate cyclosporin synthetase requires the action of other enzymes. The racemization of L-Ala to D-Ala by alanine racemase is pyridoxal phosphate-dependent. The formation of butenyl-methyl-L-threonine is performed by a Bmt polyketide synthase that uses acetate/malonate as its starting material.[36]
Gene cluster
Tolypocladium inflatum, the species currently used for mass production of Cyclosporin, has the biosynthetic genes arranged into a 12-gene cluster. Of these 12 genes, SimA (Q09164) is the cyclosporin synthetase, SimB (CAA02484.1) is the alanine racemase, and SimG (similar to ATQ39432.1) is the polyketide synthase.[37] These genes are associated with an active retrotransposon.[38] Although these sequences are poorly-annotated on GenBank and other databases, 90% similar sequences can be found for the Cyclosporin-producing Beauveria felina (or Amphichorda ~).[39] SimB has two paralogs in the same organism with different but overlapping functions thanks to their low speficity.[40]
History
In 1970, new strains of fungi were isolated from soil samples taken from Norway and from Wisconsin in the US by employees of Sandoz (now Novartis) in Basel, Switzerland. Both strains produced a family of natural products called cyclosporins. Two related components that had antifungal activity were isolated from extracts from these fungi. The Norwegian strain, Tolypocladium inflatum Gams, was later used for the large scale fermentation of ciclosporin.[41]
The immunosuppressive effect of the natural product ciclosporin was discovered in December 1971 in a screening test on immune suppression designed and implemented by Hartmann F. Stähelin at Sandoz.[42][41] The chemical structure of cyclosporin was determined in 1976, also at Sandoz.[43][44] The success of the drug candidate ciclosporin in preventing organ rejection was shown in kidney transplants by R.Y. Calne and colleagues at the University of Cambridge,[45] and in liver transplants performed by Thomas Starzl at the University of Pittsburgh Hospital. The first patient, on 9 March 1980, was a 28-year-old woman.[46] In the United States, the Food and Drug Administration (FDA) approved ciclosporin for clinical use in 1983.[47][48][49][50]
Society and culture
Name
The natural product was named cyclosporin by the German speaking scientists who first isolated it[41] and cyclosporine when translated into English. Per International Nonproprietary Name (INN) guidelines for drugs,[51] the "y" was replaced with "i" so that the INN for the medication is spelled ciclosporin.
Ciclosporin is the INN and the British Approved Name (BAN), while cyclosporine is the United States Adopted Name (USAN) and cyclosporin is a former BAN.
Cost
In 2016, the wholesale cost in the developing world was about US$106 a month.[10] In the United Kingdom, it cost the NHS about £121 per month.[11] The wholesale price in the United States was about US$173 per month.[12] In 2017, it was the 248th most commonly prescribed medication in the United States, with more than one million prescriptions that year.[13][14]
.svg.png.webp) Cyclosporine costs (US)
Cyclosporine costs (US).svg.png.webp) Cyclosporine prescriptions (US)
Cyclosporine prescriptions (US)
Available forms
Ciclosporin exhibits very poor solubility in water, and, as a consequence, suspension and emulsion forms of the medication have been developed for oral administration and for injection. Ciclosporin was originally brought to market by Sandoz (now Novartis), under the brand name Sandimmune, which is available as soft gelatin capsules, an oral solution, and a formulation for intravenous administration. These are all nonaqueous compositions.[52] A newer microemulsion,[53] orally-administered formulation, Neoral,[54] is available as a solution and as soft gelatin capsules. The Neoral compositions are designed to form microemulsions in contact with water.
Generic ciclosporin preparations have been marketed under various trade names, including Cicloral (Sandoz/Hexal), Gengraf (Abbott) and Deximune (Dexcel Pharma). Since 2002, a topical emulsion of ciclosporin for treating inflammation caused by keratoconjunctivitis sicca (dry eye syndrome) has been marketed under the trade name Restasis (0.05%). Ikervis is a similar formulation with a concentration of 0.1%.[55] Inhaled ciclosporin formulations are in clinical development, and include a solution in propylene glycol and liposome dispersions.[56][57]
Research
Neuroprotection
Ciclosporin is currently in a phase II/III (adaptive) clinical study in Europe to determine its ability to ameliorate neuronal cellular damage and reperfusion injury (phase III) in traumatic brain injury. This multi-center study is being organized by NeuroVive Pharma and the European Brain Injury Consortium using NeuroVive's formulation of ciclosporin called NeuroSTAT (also known by its cardioprotection trade name of CicloMulsion). This formulation uses a lipid emulsion base instead of cremophor and ethanol.[58] NeuroSTAT was recently compared to Sandimmune in a phase I study and found to be bioequivalent. In this study, NeuroSTAT did not exhibit the anaphylactic and hypersensitivity reactions found in cremophor- and ethanol-based products.[59]
Ciclosporin has been investigated as a possible neuroprotective agent in conditions such as traumatic brain injury, and has been shown in animal experiments to reduce brain damage associated with injury.[60] Ciclosporin blocks the formation of the mitochondrial permeability transition pore, which has been found to cause much of the damage associated with head injury and neurodegenerative diseases. Ciclosporin's neuroprotective properties were first discovered in the early 1990s when two researchers (Eskil Elmér and Hiroyuki Uchino) were conducting experiments in cell transplantation. An unintended finding was that CsA was strongly neuroprotective when it crossed the blood–brain barrier.[61] This same process of mitochondrial destruction through the opening of the MPT pore is implicated in making traumatic brain injuries much worse.[62]
Cardiac disease
Ciclosporin has been used experimentally to treat cardiac hypertrophy[26][63] (an increase in cell volume).
Inappropriate opening of the mitochondrial permeability transition pore (MPTP) manifests in ischemia[26] (blood flow restriction to tissue) and reperfusion injury[26] (damage occurring after ischemia when blood flow returns to tissue), after myocardial infarction[27] (heart attack) and when mutations in mitochondrial DNA polymerase occur.[26] The heart attempts to compensate for disease state by increasing the intracellular Ca2+
to increase the contractility cycling rates.[29] Constitutively high levels of mitochondrial Ca2+
cause inappropriate MPTP opening leading to a decrease in the cardiac range of function, leading to cardiac hypertrophy as an attempt to compensate for the problem.[29][27]
CsA (cyclosporin A) has been shown to decrease cardiac hypertrophy by affecting cardiac myocytes in many ways. CsA binds to cyclophilin D to block the opening of MPTP, and thus decreases the release of protein cytochrome C, which can cause programmed cell death.[26][29][64] CypD is a protein within the MPTP that acts as a gate; binding by CsA decreases the amount of inappropriate opening of MPTP, which decreases the intramitochondrial Ca2+
.[29] Decreasing intramitochondrial Ca2+
allows for reversal of cardiac hypertrophy caused in the original cardiac response.[29] Decreasing the release of cytochrome C caused decreased cell death during injury and disease.[26] CsA also inhibits the phosphatase calcineurin pathway (14).[26][27][65] Inhibition of this pathway has been shown to decrease myocardial hypertrophy.[27][63][65]
Veterinary use
The medication is approved in the United States for the treatment of atopic dermatitis in dogs.[66] Unlike the human form of the medication, the lower doses used in dogs mean the drug acts as an immunomodulator and has fewer side effects than in humans. The benefits of using this product include the reduced need for concurrent therapies to bring the condition under control. It is available as an ophthalmic ointment for dogs called Optimmune, manufactured by Intervet, which is part of Merck. It is also used to treat sebaceous adenitis (immune response against the sebaceous glands), pemphigus foliaceus (autoimmune blistering skin disease), Inflammatory bowel disease, anal furunculosis (anal inflammatory disease), and myasthenia gravis (a neuromuscular disease).[66][67]
It is sometimes prescribed for extreme cases of immune-mediated hemolytic anemia.[67]
See also
- Jean-François Borel
- Cremophor EL (additive in Sandimmune)
- Castor oil (additive in Sandimmune)
- Ethanol (additive in Sandimmune and Neoral)
- Voclosporin, an analog of ciclosporin
References
- ↑ "cyclosporin". Dictionary.com Unabridged. Random House. n.d. Archived from the original on 2010-11-18. Retrieved 2011-07-13.
- ↑ Laupacis A, Keown PA, Ulan RA, McKenzie N, Stiller CR (May 1982). "Cyclosporin A: a powerful immunosuppressant". Canadian Medical Association Journal. 126 (9): 1041–6. PMC 1863293. PMID 7074504.
- 1 2 3 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 221. hdl:10665/44053. ISBN 9789241547659.
- 1 2 3 4 5 6 7 "Cyclosporine". The American Society of Health-System Pharmacists. Archived from the original on 17 October 2016. Retrieved 8 December 2016.
- ↑ "Cyclosporine eent". The American Society of Health-System Pharmacists. Archived from the original on 13 January 2016. Retrieved 8 December 2016.
- ↑ "Cyclosporine Use During Pregnancy". Drugs.com. Archived from the original on 14 September 2017. Retrieved 20 December 2016.
- 1 2 Matsuda S, Koyasu S (May 2000). "Mechanisms of action of cyclosporine" (PDF). Immunopharmacology. 47 (2–3): 119–25. doi:10.1016/S0162-3109(00)00192-2. PMID 10878286. Archived from the original (PDF) on 2017-08-11. Retrieved 2018-03-04.
- ↑ Watts, Richard; Clunie, Gavin; Hall, Frances; Marshall, Tarnya (2009). Rheumatology. Oxford University Press. p. 558. ISBN 978-0-19-922999-4. Archived from the original on 2017-11-05.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "Ciclosporin". International Drug Price Indicator Guide. Archived from the original on 6 October 2018. Retrieved 8 December 2016.
- 1 2 British national formulary: BNF 69 (69th ed.). British Medical Association. 2015. p. 632. ISBN 978-0-85711-156-2.
- 1 2 "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 21 December 2016. Retrieved 20 December 2016.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Cyclosporine - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ SandImmune Label Archived 2014-04-21 at the Wayback Machine
- 1 2 "NEORAL- cyclosporine capsule, liquid filled NEORAL- cyclosporine solution". DailyMed. U.S. National Library of Medicine. Archived from the original on 2013-07-05.
- ↑ Reinhard T (2000). "Lokales Cyclosporin A bei Nummuli nach Keratoconjunctivitis epidemica Eine Pilotstudie - Springer". Der Ophthalmologe. 97 (11): 764–768. doi:10.1007/s003470070025. PMID 11130165.
- ↑ "RESTASIS - cyclosporine emulsion". DailyMed. U.S. National Library of Medicine. Archived from the original on 2014-03-30.
- ↑ Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S (June 1994). "Cyclosporine in severe ulcerative colitis refractory to steroid therapy". The New England Journal of Medicine. 330 (26): 1841–5. doi:10.1056/NEJM199406303302601. PMID 8196726.
- ↑ Naesens M, Kuypers DR, Sarwal M (February 2009). "Calcineurin inhibitor nephrotoxicity" (PDF). Clinical Journal of the American Society of Nephrology. 4 (2): 481–508. doi:10.2215/CJN.04800908. PMID 19218475. Archived (PDF) from the original on 2018-07-20. Retrieved 2018-04-20.
- ↑ Robert N, Wong GW, Wright JM (January 2010). "Effect of cyclosporine on blood pressure". Cochrane Database of Systematic Reviews (1): CD007893. doi:10.1002/14651858.CD007893.pub2. PMID 20091657.
- ↑ Lin, Hsiao-Yi; Rocher, Leslie L.; McQuillan, Mark A.; Schmaltz, Stephan; Palella, Thomas D.; Fox, Irving H. (February 1990). "Cyclosporine-induced hyperuricemia and gout". The New England Journal of Medicine. 322 (5): 334–6. doi:10.1056/NEJM199002013220514. PMID 2296276.
- ↑ Agents Classified by the IARC Monographs, Volumes 1–110 Archived 2011-10-25 at the Wayback Machine
- ↑ Humans, IARC Working Group on the Evaluation of Carcinogenic Risk to (2012). Ciclosporin. International Agency for Research on Cancer. Archived from the original on 2021-08-28. Retrieved 2018-02-23.
- ↑ Ganong, William F. (2005). "27". Review of medical physiology (22nd ed.). New York: McGraw-Hill Medical. p. 530. ISBN 978-0-07-144040-0.
- 1 2 3 4 5 6 7 8 9 10 Mott JL, Zhang D, Freeman JC, Mikolajczak P, Chang SW, Zassenhaus HP (July 2004). "Cardiac disease due to random mitochondrial DNA mutations is prevented by cyclosporin A". Biochemical and Biophysical Research Communications. 319 (4): 1210–5. doi:10.1016/j.bbrc.2004.05.104. PMID 15194495.
- 1 2 3 4 5 Youn TJ, Piao H, Kwon JS, Choi SY, Kim HS, Park DG, Kim DW, Kim YG, Cho MC (December 2002). "Effects of the calcineurin dependent signaling pathway inhibition by cyclosporin A on early and late cardiac remodeling following myocardial infarction". European Journal of Heart Failure. 4 (6): 713–8. doi:10.1016/S1388-9842(02)00120-4. PMID 12453541. Archived from the original on 2013-04-15.
- ↑ Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW (November 1984). "Cyclophilin: a specific cytosolic binding protein for cyclosporin A". Science. 226 (4674): 544–7. Bibcode:1984Sci...226..544H. doi:10.1126/science.6238408. PMID 6238408.
- 1 2 3 4 5 6 7 8 Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD (October 2010). "Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice". Journal of Clinical Investigation. 120 (10): 3680–7. doi:10.1172/JCI43171. PMC 2947235. PMID 20890047.
- ↑ Borel JF (June 2002). "History of the discovery of cyclosporin and of its early pharmacological development". Wiener Klinische Wochenschrift. 114 (12): 433–7. PMID 12422576.
Some sources list the fungus under an alternative species name Hypocladium inflatum gams such as Pritchard and Sneader in 2005:
* Pritchard DI (May 2005). "Sourcing a chemical succession for cyclosporin from parasites and human pathogens". Drug Discovery Today. 10 (10): 688–91. doi:10.1016/S1359-6446(05)03395-7. PMID 15896681.
* Sneader, Walter (2005-06-23). "Ciclosporin". Drug Discovery — A History. John Wiley & Sons. pp. 298–299. ISBN 978-0-471-89979-2.
However, the name, "Beauveria nivea", also appears in several other articles including in a 2001 online publication by Harriet Upton entitled "Origin of drugs in current use: the cyclosporin story Archived 2005-03-08 at the Wayback Machine" (retrieved June 19, 2005). Mark Plotkin states in his book Medicine Quest, Penguin Books 2001, pages 46-47, that in 1996 mycology researcher Kathie Hodge found Archived 2006-03-10 at the Wayback Machine that it is in fact a species of Cordyceps. - ↑ Wang CP, Hartman NR, Venkataramanan R, Jardine I, Lin FT, Knapp JE, Starzl TE, Burckart GJ (1989). "Isolation of 10 cyclosporine metabolites from human bile". Drug Metabolism and Disposition. 17 (3): 292–6. PMC 3154783. PMID 2568911.
- ↑ Copeland KR, Yatscoff RW, McKenna RM (February 1990). "Immunosuppressive activity of cyclosporine metabolites compared and characterized by mass spectroscopy and nuclear magnetic resonance". Clinical Chemistry. 36 (2): 225–9. doi:10.1093/clinchem/36.2.225. PMID 2137384.
- ↑ Lawen A (October 2015). "Biosynthesis of cyclosporins and other natural peptidyl prolyl cis/trans isomerase inhibitors". Biochimica et Biophysica Acta (BBA) - General Subjects. 1850 (10): 2111–20. doi:10.1016/j.bbagen.2014.12.009. PMID 25497210.
- ↑ Dittmann J, Wenger RM, Kleinkauf H, Lawen A (January 1994). "Mechanism of cyclosporin A biosynthesis. Evidence for synthesis via a single linear undecapeptide precursor". Journal of Biological Chemistry. 269 (4): 2841–6. PMID 8300618.
- ↑ Hoppert M, Gentzsch C, Schörgendorfer K (October 2001). "Structure and localization of cyclosporin synthetase, the key enzyme of cyclosporin biosynthesis in Tolypocladium inflatum" (PDF). Archives of Microbiology. 176 (4): 285–93. doi:10.1007/s002030100324. PMID 11685373.
- ↑ Dewick, P. (2001) Medicinal Natural Products. John Wiley & Sons, Ltd. 2nd ed.
- ↑ Yang X, Feng P, Yin Y, Bushley K, Spatafora JW, Wang C (October 2018). "Tolypocladium inflatum Benefits Fungal Adaptation to the Environment". mBio. 9 (5). doi:10.1128/mBio.01211-18. PMC 6168864. PMID 30279281.
- ↑ Bushley KE, Raja R, Jaiswal P, Cumbie JS, Nonogaki M, Boyd AE, Owensby CA, Knaus BJ, Elser J, Miller D, Di Y, McPhail KL, Spatafora JW (June 2013). "The genome of tolypocladium inflatum: evolution, organization, and expression of the cyclosporin biosynthetic gene cluster". PLOS Genetics. 9 (6): e1003496. doi:10.1371/journal.pgen.1003496. PMC 3688495. PMID 23818858.
- ↑ Xu L, Li Y, Biggins JB, Bowman BR, Verdine GL, Gloer JB, Alspaugh JA, Bills GF (March 2018). "Identification of cyclosporin C from Amphichorda felina using a Cryptococcus neoformans differential temperature sensitivity assay". Applied Microbiology and Biotechnology. 102 (5): 2337–2350. doi:10.1007/s00253-018-8792-0. PMC 5942556. PMID 29396588.
- ↑ di Salvo ML, Florio R, Paiardini A, Vivoli M, D'Aguanno S, Contestabile R (January 2013). "Alanine racemase from Tolypocladium inflatum: a key PLP-dependent enzyme in cyclosporin biosynthesis and a model of catalytic promiscuity". Archives of Biochemistry and Biophysics. 529 (2): 55–65. doi:10.1016/j.abb.2012.11.011. PMID 23219598.
- 1 2 3 Borel JF, Kis ZL, Beveridge T (1995). "The history of the discovery and development of Cyclosporin (Sandimmune®)". In Merluzzi VJ, Adams J (eds.). The search for anti-inflammatory drugs case histories from concept to clinic. Boston: Birkhäuser. pp. 27–63. ISBN 978-1-4615-9846-6. Archived from the original on 2017-11-05.
- ↑ Borel JF, Feurer C, Gubler HU, Stähelin H (July 1976). "Biological effects of cyclosporin A: a new antilymphocytic agent". Agents and Actions. 6 (4): 468–75. doi:10.1007/bf01973261. PMID 8969.
- ↑ Rüegger A, Kuhn M, Lichti H, Loosli HR, Huguenin R, Quiquerez C, von Wartburg A (1976). "[Cyclosporin A, a Peptide Metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity]" [Cyclosporin A, a Peptide Metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity]. Helvetica Chimica Acta (in German). 59 (4): 1075–92. doi:10.1002/hlca.19760590412. PMID 950308.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Heusler K, Pletscher A (June 2001). "The controversial early history of cyclosporin". Swiss Medical Weekly. 131 (21–22): 299–302. PMID 11584691. Archived from the original on 2017-09-02. Retrieved 2019-01-10.
- ↑ Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Pentlow BD, Rolles K (1978). "Cyclosporin A in patients receiving renal allografts from cadaver donors". The Lancet. 2 (8104–5): 1323–7. doi:10.1016/S0140-6736(78)91970-0. PMID 82836.
- ↑ Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schröter GP (July 1981). "Liver transplantation with use of cyclosporin a and prednisone". The New England Journal of Medicine. 305 (5): 266–9. doi:10.1056/NEJM198107303050507. PMC 2772056. PMID 7017414.
- ↑ Kolata G (September 1983). "FDA speeds approval of cyclosporin". Science. 221 (4617): 1273. Bibcode:1983Sci...221.1273K. doi:10.1126/science.221.4617.1273-a. PMID 17776314.
On 2 September (1983), the Food and Drug Administration approved cyclosporin, a new drug that suppresses the immune system.
- ↑ Gottesman, Jill (20 March 1988). "Milestones in Cardiac Care". Los Angeles Times. Archived from the original on 26 February 2017.
- ↑ "First Successful Pediatric Heart Transplant [9 June 1984]". Columbia University Medical Center, Dept. of Surgery, Cardiac Transplant Program. Archived from the original on 1 March 2017.
It [cyclosporine] gained FDA approval at the end of 1983, ...
- ↑ "Drugs@FDA: FDA Approved Drug Products [Click on "Approval Date(s) and History]". United States Food and Drug Administration. Archived from the original on 2017-03-01.
Drug Name(s): Sandimmune (Cyclosporine), Company: Novartis, Action Date: 11/14/1983, Action Type: Approval, Submission Classification: Type 1 - New Molecular Entity, Review Priority: Priority
- ↑ "Guidelines on the Use of International Nonproprietary Names (INNs) for Pharmaceutical Substances". World Health Organization. 1997.
To facilitate the translation and pronunciation of INN, “f” should be used instead of “ph”, “t” instead of “th”, “e” instead of “ae” or “oe”, and “i” instead of “y”; the use of the letters “h” and “k” should be avoided.
- ↑ Sandimmune Prescribing Information Archived 2004-07-19 at the Wayback Machine
- ↑ Gibaud S, Attivi D (August 2012). "Microemulsions for oral administration and their therapeutic applications". Expert Opinion on Drug Delivery. 9 (8): 937–51. doi:10.1517/17425247.2012.694865. PMID 22663249. Archived from the original on 2018-03-05. Retrieved 2018-03-04.
- ↑ Neoral Prescribing Information Archived 2007-07-28 at the Wayback Machine
- ↑ "Ikervis". Santen. Archived from the original on 2018-07-03. Retrieved 2018-07-03.
- ↑ Clinical trial number NCT01287078 for "Cyclosporine Inhalation Solution (CIS) in Lung Transplant and Hematopoietic Stem Cell Transplant Recipients for the Treatment of Bronchiolitis Obliterans" at ClinicalTrials.gov.
- ↑ Trammer B, Amann A, Haltner-Ukomadu E, Tillmanns S, Keller M, Högger P (November 2008). "Comparative permeability and diffusion kinetics of cyclosporine A liposomes and propylene glycol solution from human lung tissue into human blood ex vivo". European Journal of Pharmaceutics and Biopharmaceutics. 70 (3): 758–64. doi:10.1016/j.ejpb.2008.07.001. PMID 18656538.
- ↑ Administrator. "Hem - NeuroVive Pharmaceutical AB". neurovive.com. Archived from the original on 2014-01-06.
- ↑ Ehinger KH, Hansson MJ, Sjövall F, Elmér E (January 2013). "Bioequivalence and tolerability assessment of a novel intravenous ciclosporin lipid emulsion compared to branded ciclosporin in Cremophor ® EL" (PDF). Clinical Drug Investigation. 33 (1): 25–34. doi:10.1007/s40261-012-0029-x. PMC 3586182. PMID 23179472. Archived (PDF) from the original on 2018-10-26. Retrieved 2018-10-26.
- ↑ Sullivan PG, Thompson M, Scheff SW (February 2000). "Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury". Experimental Neurology. 161 (2): 631–7. doi:10.1006/exnr.1999.7282. PMID 10686082.
- ↑ Uchino H, Elmér E, Uchino K, Lindvall O, Siesjö BK (December 1995). "Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat". Acta Physiologica Scandinavica. 155 (4): 469–71. doi:10.1111/j.1748-1716.1995.tb09999.x. PMID 8719269.
- ↑ Sullivan PG, Sebastian AH, Hall ED (February 2011). "Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury". Journal of Neurotrauma. 28 (2): 311–8. doi:10.1089/neu.2010.1646. PMC 3037811. PMID 21142667.
- 1 2 Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ (November 1998). "Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways". Proceedings of the National Academy of Sciences of the United States of America. 95 (23): 13893–8. Bibcode:1998PNAS...9513893M. doi:10.1073/pnas.95.23.13893. PMC 24952. PMID 9811897.
- ↑ Wilkinson ST, Johnson DB, Tardif HL, Tome ME, Briehl MM (March 2010). "Increased cytochrome c correlates with poor survival in aggressive lymphoma". Oncology Letters. 1 (2): 227–230. doi:10.3892/ol_00000040. PMC 2927837. PMID 20798784.
- 1 2 Lim HW, De Windt LJ, Mante J, Kimball TR, Witt SA, Sussman MA, Molkentin JD (April 2000). "Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition". Journal of Molecular and Cellular Cardiology. 32 (4): 697–709. doi:10.1006/jmcc.2000.1113. PMID 10756124.
- 1 2 Archer TM, Boothe DM, Langston VC, Fellman CL, Lunsford KV, Mackin AJ (2014). "Oral cyclosporine treatment in dogs: a review of the literature". Journal of Veterinary Internal Medicine. 28 (1): 1–20. doi:10.1111/jvim.12265. PMC 4895546. PMID 24341787.
- 1 2 Palmeiro BS (January 2013). "Cyclosporine in veterinary dermatology". Veterinary Clinics of North America: Small Animal Practice. 43 (1): 153–71. doi:10.1016/j.cvsm.2012.09.007. PMID 23182330.
External links
| Identifiers: |
|---|
- Cyclosporine at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Cyclosporine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2017-09-30. Retrieved 2016-12-09.
- ChemSub Online : Cyclosporin A Archived 2017-09-30 at the Wayback Machine