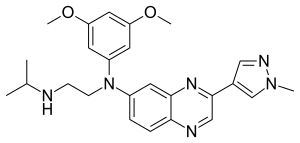
Erdafitinib
 | |
| Names | |
|---|---|
| Trade names | Balversa |
| Other names | JNJ-42756493 |
IUPAC name
| |
| Clinical data | |
| Drug class | Fibroblast growth factor receptor (FGFR) blocker[1] |
| Main uses | Urothelial cancer[2] |
| Side effects | High phosphate, mouth inflammation, tiredness, kidney problems, diarrhea, liver problems, change in taste, low magnesium, hair loss, high calcium, muscle pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 8 to 9 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619031 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C25H30N6O2 |
| Molar mass | 446.555 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Erdafitinib, sold under the brand name Balversa, is a medication used to treat urothelial cancer.[2] Specifically it is used for cases which have suitable FGFR-2 or 3 mutations.[2] It is taken by mouth.[2]
Common side effects include high phosphate, mouth inflammation, tiredness, kidney problems, diarrhea, liver problems, change in taste, low magnesium, hair loss, high calcium, and muscle pain.[2] Other side effects may include infertility and vision problems.[2] Use in pregnancy may harm the baby.[2] It is a tyrosine kinase inhibitor, specifically a fibroblast growth factor receptor (FGFR) blocker.[1][2]
Erdafitinib was approved for medical use in the United States in 2019.[2] It is not approved for use in the United Kingdom or Europe as of 2021.[1] In the United States 4 weeks of treatment costs about 21,500 USD as of 2021.[3]
Medical uses
Dosage
Erdafitinib is a tablet taken once a day for a total dose of 8 mg. During treatment, the erdafitinib dose may be increased to 9 mg if needed.[4]
Side effects
Common side effects include increased phosphate level, mouth sores, feeling tired, change in kidney function, diarrhea, dry mouth, nails separating from the bed or poor formation of the nail, change in liver function, low salt (sodium) levels, decreased appetite, change in sense of taste, low red blood cells (anemia), dry skin, dry eyes and hair loss.[5] Other side effects include redness, swelling, peeling or tenderness on the hands or feet (hand foot syndrome), constipation, stomach pain, nausea and muscle pain.[5]
Erdafitinib may cause serious eye problems, including inflamed eyes, inflamed cornea (front part of the eye) and disorders of the retina, an internal part of the eye.[5] Patients are advised to have eye examinations intermittently and to tell their health care professional right away if they develop blurred vision, loss of vision or other visual changes.[5]
History
The efficacy of erdafitinib was studied in a clinical trial (NCT02365597) that included 87 adults with locally advanced or metastatic bladder cancer, with FGFR3 or FGFR2 genetic alterations, that had progressed following treatment with chemotherapy.[5][4] The overall response rate in these adults was 32.2%, with 2.3% having a complete response and almost 30% having a partial response.[5] The response lasted for an average of approximately five-and-a-half months.[5] The trial was conducted in Asia, Europe, and the United States.[4]
Erdafitinib received an accelerated approval.[5] Further clinical trials are required to confirm erdafitinib's clinical benefit and the sponsor is conducting or plans to conduct these studies.[5] Erdafitinib was also granted breakthrough therapy designation.[5]
The FDA granted the approval of Balversa to Janssen Pharmaceutical.[5] The FDA also approved the therascreen FGFR RGQ RT-PCR Kit, developed by Qiagen Manchester, Ltd., for use as a companion diagnostic with Balversa for this therapeutic indication.[5]
History
Researchers have investigated erdafitinib for safety and efficacy in treatment of bile duct cancer, gastric cancer, non-small cell lung cancer, and esophageal cancer.[6]
In March 2018, erdafitinib was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for treatment of urothelial cancer.[7]
In April 2019, erdafitinib was granted approval by the FDA for treatment of metastatic or locally advanced bladder cancer with an FGFR3 or FGFR2 alteration that has progressed beyond traditional platinum-based therapies, subject to a confirmatory trial.[5][8] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[9]
References
- 1 2 3 "Erdafitinib". SPS - Specialist Pharmacy Service. 5 October 2018. Archived from the original on 11 January 2022. Retrieved 15 December 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Erdafitinib Monograph for Professionals". Drugs.com. Archived from the original on 3 January 2022. Retrieved 15 December 2021.
- ↑ "Erdafitinib Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 15 December 2021.
- 1 2 3 "Drug Trials Snapshots: Balversa". U.S. Food and Drug Administration (FDA). 12 April 2019. Archived from the original on 27 September 2019. Retrieved 24 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 6 7 8 9 10 11 12 13 "FDA approves first targeted therapy for metastatic bladder cancer". U.S. Food and Drug Administration (FDA) (Press release). 12 April 2019. Archived from the original on 15 November 2019. Retrieved 13 May 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, Awad M, Calvo E, Moreno V, Govindan R, Spira A, Gonzalez M, Zhong B, Santiago-Walker A, Poggesi I, Parekh T, Xie H, Infante J, Tabernero J (August 2019). "Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors". Clin. Cancer Res. 25 (16): 4888–97. doi:10.1158/1078-0432.CCR-18-3334. PMID 31088831. S2CID 155089088.
- ↑ "Janssen Announces U.S. FDA Breakthrough Therapy Designation for Erdafitinib in the Treatment of Metastatic Urothelial Cancer". Johnson & Johnson (Press release). Archived from the original on 20 June 2018.
- ↑ "Balversa (erdafitinib) Receives U.S. FDA Approval for the Treatment of Patients with Locally Advanced or Metastatic Urothelial Carcinoma with Certain FGFR Genetic Alterations". Johnson & Johnson (Press release). 8 May 2019. Archived from the original on 8 May 2019. Retrieved 24 November 2019.
- ↑ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Archived from the original on 16 September 2020. Retrieved 15 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Drug Approval Package: Balversa (erdafinitib)". U.S. Food and Drug Administration (FDA). Archived from the original on 31 March 2021. Retrieved 1 July 2021.