Tivozanib
 | |
| Names | |
|---|---|
| Trade names | Fotivda |
| Other names | AV-951 |
| Clinical data | |
| Main uses | Renal cell carcinoma (RCC)[1] |
| Side effects | Tiredness, high blood pressure, diarrhea, nausea, change in voice, low thyroid, cough, mouth inflammation[1] |
| Interactions | St John’s wort[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | >99% |
| Elimination half-life | 4.5–5.1 days |
| Excretion | 79% faeces, 12% urine |
| Chemical and physical data | |
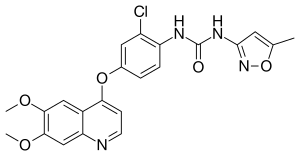
| Formula | C22H19ClN4O5 |
| Molar mass | 454.87 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tivozanib, sold under the brand name Fotivda, is a medication used to treat renal cell carcinoma (RCC).[1] Specifically it is used for advanced disease which has failed other treatments.[1] It is taken by mouth.[1]
Common side effects include tiredness, high blood pressure, diarrhea, nausea, change in voice, low thyroid, cough, and mouth inflammation.[1] Other side effects may include low sodium, heart problems, blood clots, bleeding, and increased lipase.[1] Use in pregnancy may harm the baby.[1] It is a VEGF receptor tyrosine kinase inhibitor, which blocks the formation of new blood vessels.[2][1]
Tivozanib was approved for medical use in Europe in 2017 and the United States in 2021.[2][1] In the United States 4 weeks of treatment costs about 26,000 USD.[4]
Medical uses
Tivozanib is used for the treatment of adults with relapsed or refractory advanced renal cell carcinoma (RCC) following two or more prior systemic therapies.[3]
It increases the time to the disease worsening by 12 month as opposed to 9 months with sorafenib.[2]
Dosage
It is taken as 1.34 mg once per day for 21 days followed by 7 days without; which is than repeated.[1] For those with liver problems a dose of 0.89 mg per day may be used.[1]
Side effects
The most common side effects in studies were hypertension (high blood pressure, in 48% of patients), dysphonia (hoarse voice, 27%), fatigue and diarrhoea (both 26%). A hypertensive crisis occurred in 1% of patients.[5]
Interactions
Administration of a single dose of tivozanib with rifampicin, a strong inducer of the enzyme CYP3A4, cuts the biological half-life and total exposure (AUC) of tivozanib in half, but has no relevant influence on highest concentrations in the blood. Combination with ketoconazole, a strong CYP3A4 inhibitor, has no relevant effects. The clinical significance of these findings is not known.[5]
Tivozanib must not be combined with St. John's Wort, an inducer of the liver enzyme CYP3A4 (see interactions below). It should not be taken during pregnancy as it is teratogenic, embryotoxic and fetotoxic in rats.[5]
Pharmacology
Mechanism of action
A quinoline urea derivative, tivozanib suppresses angiogenesis by being selectively inhibitory against vascular endothelial growth factor (VEGF).[6] It is designed to inhibit all three VEGF receptors.[7]
Pharmacokinetics
After tivozanib is taken by mouth, highest blood serum levels are reached after 2 to 24 hours. The total AUC is independent of food intake. When in the bloodstream, over 99% of the substance are bound to plasma proteins, predominantly albumin. Although the enzymes CYP3A4 and CYP1A1 and several UGTs are capable of metabolising the drug, over 90% circulate in unchanged form. The metabolites are demethylation, hydroxylation and N-oxidation products and glucuronides.[5]
The biological half-life is 4.5 to 5.1 days; 79% being excreted via the faeces, mostly unchanged, and 12% via the urine, completely unchanged.[5]
Chemistry
Tivozanib is used in form of the hydrochloride monohydrate, which is a white to light brown powder. It is practically insoluble in water and has low solubility in aqueous acids, ethanol and methanol. It is not hygroscopic and not optically active.[8]
History
It was discovered by Kyowa Kirin and developed by AVEO Pharmaceuticals.[9]
Clinical trials
Phase III results on advanced renal cell carcinoma suggested a 30% or 3 months improvement in median progression-free survival compared to sorafenib but showed an inferior overall survival rate of the experimental arm versus the control arm.[7][10] The Food and Drug Administration's Oncologic Drugs Advisory Committee voted in May 2013 13 to 1 against recommending approval of tivozanib for renal cell carcinoma. The committee felt the drug failed to show a favorable risk-benefit ratio and questioned the equipose of the trial design, which allowed control arm patients who used sorafenib to transition to tivozanib following progression disease but not those on the experimental arm using tivozanib to transition to sorafenib. The application was formally rejected by the FDA in June 2013, saying that approval would require additional clinical studies.[10]
In 2016, AVEO Oncology published data in conjunction with the ASCO meeting showing a geographical location effect on overall survival in the Phase III trial.[11]
In 2016, AVEO Oncology announced the start of a second Phase III clinical study in third line advanced RCC patients.[12]
In 2016, EUSA Pharma and AVEO Oncology announced that tivozanib had been submitted to the European Medicines Agency for review under the centralised procedure.[13]
In June 2017, the EMA Scientific Committee recommended tivozanib for approval in Europe, with approval expected in September.[14]
In August 2017, the European Commission (EC) formally approved tivozanib in Europe.[15]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Fotivda- tivozanib capsule". DailyMed. Archived from the original on 13 September 2021. Retrieved 12 September 2021.
- 1 2 3 4 5 "Fotivda EPAR". European Medicines Agency (EMA). Archived from the original on 13 May 2021. Retrieved 16 March 2021.
- 1 2 "FDA approves tivozanib for relapsed or refractory advanced renal cell". U.S. Food and Drug Administration. 10 March 2021. Archived from the original on 11 March 2021. Retrieved 16 March 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Tivozanib". Archived from the original on 31 October 2022. Retrieved 31 October 2022.
- 1 2 3 4 5 "Fotivda: EPAR – Product Information" (PDF). European Medicines Agency. 2017-11-22. Archived (PDF) from the original on 2018-06-18. Retrieved 2021-09-13.
- ↑ Campas C, Bolos J, Castaner R (October 2009). "Tivozanib VEGFR Tyrosine Kinase Inhibitor Angiogenesis Inhibitor Oncolytic". Drugs of the Future. 34 (10): 793–6. doi:10.1358/dof.2009.034.10.1417872. Archived from the original on 2019-12-15. Retrieved 2021-09-13.
- 1 2 "Phase III Results Lead Aveo and Astellas to Plan Regulatory Submissions for Tivozanib". 3 Jan 2012. Archived from the original on 18 June 2018. Retrieved 13 September 2021.
- ↑ "Fotivda: EPAR – Public assessment report" (PDF). European Medicines Agency. 2017-11-22. Archived (PDF) from the original on 2018-06-18. Retrieved 2021-09-13.
- ↑ "Tivozanib (VEGFR TKI)". AVEO Pharmaceuticals, Inc. Archived from the original on 2021-08-04. Retrieved 2021-09-13.
- 1 2 "FDA Rejects Renal Cancer Drug Tivozanib". MedPage Today. June 30, 2013. Archived from the original on May 10, 2019. Retrieved September 13, 2021.
- ↑ Needle MN, Hutson TE, Motzer RJ (2016). "The effect of geography and the availability of second-line therapy on overall survival in a one-way crossover design study in renal cell carcinoma". J Clin Oncol. 34 (supplement): abstr e16120). doi:10.1200/JCO.2016.34.15_suppl.e16120. Archived from the original on 2016-09-19. Retrieved 2021-09-13.
- ↑ "AVEO Announces Dosing of First Patient in the Pivotal Phase 3 TIVO-3 Study of Tivozanib in Renal Cell Carcinoma" (Press release). AVEO Pharmaceuticals, Inc. Archived from the original on 2016-06-11. Retrieved 2016-07-08.
- ↑ "EUSA Pharma and AVEO Announce Submission of Marketing Authorization Application for Tivozanib in Advanced Renal Cell Carcinoma" (PDF). EUSA Pharma. Archived from the original (PDF) on 2016-04-29. Retrieved 2016-07-08.
- ↑ "AVEO Pharma surges 48% on recommendation for European approval of its cancer drug". Market Watch. June 28, 2017. Archived from the original on October 3, 2017. Retrieved June 28, 2017.
- ↑ "AVEO Oncology Announces Fotivda (tivozanib) Approved in the European Union for the Treatment of Advanced Renal Cell Carcinoma" (PDF). AVEO Oncology (Press release). August 28, 2017. Archived from the original (PDF) on June 21, 2019. Retrieved February 9, 2018.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|