Trofinetide
 | |
| Names | |
|---|---|
| Trade names | Daybue |
| Other names | NNZ-2566 |
IUPAC name
| |
| Clinical data | |
| Main uses | Rett syndrome[1] |
| Side effects | Diarrhea, vomiting[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 5,000 to 12,000 mg BID[1] |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 84% |
| Metabolism | Insignificant |
| Elimination half-life | ~ 1.5 h |
| Excretion | Urine |
| Chemical and physical data | |
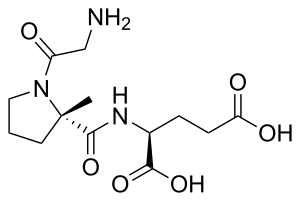
| Formula | C13H21N3O6 |
| Molar mass | 315.326 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Trofinetide, sold under the brand name Daybue, is a medication used to treat Rett syndrome.[1] It is used in people at least two years old.[1] It is taken by mouth.[1]
Common side effects include diarrhea and vomiting.[1] Weight loss may occur.[1] use is not recommended in those with significant kidney problems.[1] How it works is unclear.[1]
Trofinetide was approved for medical use in the United States in 2023.[1] For those who weight less than 12 kg it will cost about 385,000 USD per year, while in those who more than 50 kg it will cost about 924,000 USD per year in the United States as of 2023.[2]
Medical uses
Trofinetide is indicated for the treatment of Rett syndrome in people two years of age and older.[1]
Rett syndrome is a rare, genetic neurological and developmental disorder that affects the way the brain develops.[3] People experience a progressive loss of motor skills and language.[3] Most babies seem to develop as expected for the first six months of life.[3] These babies then lose skills they previously had attained at approximately six to 18 months of age — such as the ability to crawl, walk, communicate, or use their hands.[3] The hallmark is near constant repetitive hand movements, such as rubbing or clapping.[3] Rett syndrome leads to severe impairments affecting nearly every aspect of life, including the ability to speak, walk, eat, and breathe.[3]
Dosage
It is given twice per day at doses of 5,000 to 12,000 mg based on a persons weight.[1]
History
It was developed by Neuren Pharmaceuticals that acts as an analogue of the neuropeptide (1-3) IGF-1, which is a simple tripeptide with sequence Gly-Pro-Glu obtained by enzymatic cleavage of the growth factor IGF-1 within the brain. Trofinetide has anti-inflammatory properties and was originally developed as a potential treatment for stroke,[4][5] but has subsequently been developed for other applications and is now approved by the FDA as an oral solution. It has successfully completed Phase III clinical trial against Rett syndrome.[6] Trofinetide has also had a successful Phase II trial against Fragile X syndrome.[7][8][9] The drug is manufactured by Acadia Pharmaceuticals.
The US Food and Drug Administration (FDA) evaluated the efficacy and safety of trofinetide based on a randomized, double-blind, placebo-controlled, 12-week study (Study 1; NCT04181723) of participants with Rett syndrome five to 20 years of age.[3] Participants were randomized to receive trofinetide (N=93) or matching placebo (N=94) for 12 weeks.[3] The dose of trofinetide was based on participant weight to achieve similar exposure in all participants.[3]
The FDA granted the application for trofinetide priority review, orphan drug, and fast track designations.[3]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "DailyMed - DAYBUE- trofinetide solution". dailymed.nlm.nih.gov. Archived from the original on 2 July 2023. Retrieved 12 June 2023.
- ↑ "FDA Approves First Treatment for Rett syndrome". Formulary Watch. 13 March 2023. Archived from the original on 22 May 2023. Retrieved 12 June 2023.
- 1 2 3 4 5 6 7 8 9 10 "FDA approves first treatment for Rett Syndrome". U.S. Food and Drug Administration (FDA). 13 March 2023. Archived from the original on 13 March 2023. Retrieved 13 March 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Bickerdike MJ, Thomas GB, Batchelor DC, Sirimanne ES, Leong W, Lin H, et al. (March 2009). "NNZ-2566: a Gly-Pro-Glu analogue with neuroprotective efficacy in a rat model of acute focal stroke". Journal of the Neurological Sciences. 278 (1–2): 85–90. doi:10.1016/j.jns.2008.12.003. PMID 19157421. S2CID 7789415.
- ↑ Cartagena CM, Phillips KL, Williams GL, Konopko M, Tortella FC, Dave JR, Schmid KE (September 2013). "Mechanism of action for NNZ-2566 anti-inflammatory effects following PBBI involves upregulation of immunomodulator ATF3". Neuromolecular Medicine. 15 (3): 504–14. doi:10.1007/s12017-013-8236-z. PMID 23765588. S2CID 12522580. Archived from the original on 2023-04-15. Retrieved 2023-04-14.
- ↑ "Positive top-line results from pivotal Phase 3 trial in Rett syndrome" (PDF). Rettsyndrome.org. 7 December 2021. Archived (PDF) from the original on 18 August 2022. Retrieved 21 July 2022.
- ↑ Deacon RM, Glass L, Snape M, Hurley MJ, Altimiras FJ, Biekofsky RR, Cogram P (March 2015). "NNZ-2566, a novel analog of (1-3) IGF-1, as a potential therapeutic agent for fragile X syndrome". Neuromolecular Medicine. 17 (1): 71–82. doi:10.1007/s12017-015-8341-2. PMID 25613838. S2CID 11964380.
- ↑ "Study Details - Rett Syndrome Study". Rettstudy.com. Archived from the original on 4 October 2016. Retrieved 21 July 2022.
- ↑ "Neuren's Tofinetide Successful in Phase 2 Clinical Trial in Fragile X". Fraxa.org. 7 December 2015. Archived from the original on 24 June 2022. Retrieved 21 July 2022.
External links
| Identifiers: |
|---|
- Clinical trial number NCT04181723 for "Study of Trofinetide for the Treatment of Girls and Women With Rett Syndrome (LAVENDER)" at ClinicalTrials.gov