R7 (drug)
 | |
| Clinical data | |
|---|---|
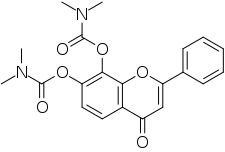
| Other names | 4-Oxo-2-phenyl-4H-chromene-7,8-diyl bis(dimethylcarbamate) |
| Routes of administration | By mouth[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~35% (in mice)[2] |
| Metabolites | Tropoflavin[1] |
| Elimination half-life | ~3.25 hours (in mice[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C21H20N2O6 |
| Molar mass | 396.399 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
R7 is a small-molecule flavonoid and orally active, potent, and selective agonist of the tropomyosin receptor kinase B (TrkB) – the main signaling receptor for the neurotrophin brain-derived neurotrophic factor (BDNF) – which is under development for the treatment of Alzheimer's disease.[1][3][2] It is a structural modification and prodrug of tropoflavin (7,8-DHF) with improved potency and pharmacokinetics, namely oral bioavailability and duration.[2] R7 was synthesized by the same researchers who were involved in the discovery of tropoflavin.[2][4] A patent was filed for R7 in 2013 and was published in 2015.[2] In 2016, it was reported to be in the preclinical stage of development.[1][3] R7 was superseded by R13 because while R7 had a good drug profile in animals, it showed almost no conversion into tropoflavin in human liver microsomes.[5]
In 2010, tropoflavin, a naturally occurring flavonoid, was found to act as an agonist of the TrkB with nanomolar affinity (Kd ≈ 320 nM).[4] Subsequently, tropoflavin demonstrated robust efficacy in animal models of Alzheimer's disease and a variety of other conditions, making it a highly promising potential therapeutic agent.[1] Due to the presence of a vulnerable catechol group on its 2-phenyl-4H-chromene ring, tropoflavin is extensively conjugated via glucuronidation, sulfation, and methylation during first-pass metabolism in the liver and has a poor oral bioavailability of only 5% in mice upon oral administration.[2] As such, tropoflavin itself is a poor candidate for clinical development as an oral medication.[2] R7 is a derivative of tropoflavin with carbamate moieties on its hydroxyl groups, thereby protecting it from metabolism.[2]
As R7 is a slightly larger molecule than tropoflavin, 72.5 mg R7 is molecularly equivalent to 50 mg tropoflavin.[2] Relative to a roughly molecularly equivalent dose of tropoflavin, the area-under-curve levels of R7 were found to be 7.2-fold higher upon oral administration to mice, and R7 hence has a greatly improved oral bioavailability in mice of approximately 35%.[2] Moreover, whereas tropoflavin itself is mostly metabolized in mice within 30 minutes, tropoflavin as a metabolite was still detectable in plasma at 8 hours after administration with R7, indicating that R7 sustainably releases tropoflavin into circulation.[2] In accordance, the terminal half-life of R7 is about 195 minutes (3.25 hours) in mice.[2] The Tmax of R7 is about 60 minutes in mice, and its Cmax for a 78 mg/kg dose was 262 ng/mL, whereas that for a 50 mg/kg dose of tropoflavin was 70 ng/mL.[2]
Like tropoflavin, administration of R7 has been found to activate the TrkB in vivo in the mouse brain.[2] Moreover, R7 was found to potently activate the TrkB and the downstream Akt signaling pathway upon oral administration, an action that was tightly correlated with plasma concentrations of tropoflavin.[2] As such, R7 has shown in vivo efficacy as an agonist of the TrkB, including central activity, similarly to tropoflavin.[2]
See also
- List of investigational antidepressants
- Tropomyosin receptor kinase B § Agonists
References
- 1 2 3 4 5 Liu, Chaoyang; Chan, Chi Bun; Ye, Keqiang (2016). "7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders". Translational Neurodegeneration. 5 (1): 2. doi:10.1186/s40035-015-0048-7. ISSN 2047-9158. PMC 4702337. PMID 26740873.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 US application 20150274692, Keqiang Ye, "7,8-Dihydoxyflavone and 7,8-substituted flavone derivatives, compositions, and methods related thereto", published 2015-10-01, assigned to Emory University
- 1 2 Kazim, Syed Faraz; Iqbal, Khalid (2016). "Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer's disease". Molecular Neurodegeneration. 11 (1): 50. doi:10.1186/s13024-016-0119-y. ISSN 1750-1326. PMC 4940708. PMID 27400746.
- 1 2 Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K (2010). "A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone". Proc. Natl. Acad. Sci. U.S.A. 107 (6): 2687–92. Bibcode:2010PNAS..107.2687J. doi:10.1073/pnas.0913572107. PMC 2823863. PMID 20133810.
- ↑ Chen C, Wang Z, Zhang Z, Liu X, Kang SS, Zhang Y, Ye K (January 2018). "The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer's disease". Proc. Natl. Acad. Sci. U.S.A. 115 (3): 578–583. doi:10.1073/pnas.1718683115. PMC 5777001. PMID 29295929.
External links