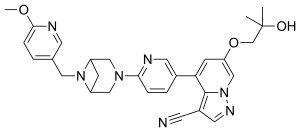
Selpercatinib
 | |
| Names | |
|---|---|
| Trade names | Retevmo, Retsevmo |
| Other names | LOXO-292 |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor |
| Main uses | Non-small cell lung cancer, thyroid cancer[1] |
| Side effects | Liver problems, high blood sugar, dry mouth, diarrhea, kidney problems, tiredness, swelling, low platelets, rash[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 160 mg BID[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620036 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C29H31N7O3 |
| Molar mass | 525.613 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Selpercatinib, sold under the brand name Retevmo, is a medication used to treat certain types of non-small cell lung cancer and thyroid cancer.[1] It is used in cases that are either RET fusion positive or have a RET gene mutation.[1][2] It is used after other treatments have failed.[5] It is taken by mouth.[2]
Common side effects include liver problems, high blood sugar, dry mouth, diarrhea, kidney problems, tiredness, swelling, low platelets, and rash.[2] Other side effects may include high blood pressure, QT prolongation, bleeding, allergic reactions, and tumor lysis syndrome.[2] Use in pregnancy may harm the baby.[2] It is a kinase inhibitor, which blocks RET.[2]
Selpercatinib was approved for medical use in the United States in 2020 and Europe in 2021.[1][3] In the United States it costs about 258,000 USD per year as of 2021.[6] It has also bee approved in the United Kingdom.[5]
Medical uses
Selpercatinib is a kinase inhibitor indicated for the treatment of
- adults with non-small cell lung cancer (NSCLC) which has spread to other parts of the body (metastatic).[7][2][8]
- adults and children twelve years of age and older with advanced or metastatic medullary thyroid cancer (MTC).[7][2][8]
- adults and children twelve years of age and older with advanced or metastatic thyroid cancer who have received radioactive iodine that did not work or is no longer working.[7][2][8]
Dosage
It is generally taken at a dose of 160 mg twice per day.[3]
Side effects
Selpercatinib can cause serious side effects including liver toxicity, high blood pressure, heart rhythm changes due to prolongation of heart electrical activity (QT prolongation), bleeding, allergic reactions, impaired wound healing and harm to an unborn baby.[7][2][8]
Selpercatinib may cause harm to a developing fetus or a newborn baby.[7][2]
History
Selpercatinib was approved for medical use in the United States in May 2020.[7][9][10][8] Selpercatinib is the first therapy approved specifically for people with cancer and the RET gene alterations.[7]
Selpercatinib was approved based on the results of the LIBRETTO-001 clinical trial (NCT03157128) involving 702 participants aged 15–92 years with each of the three types of tumors.[7][10][8] During the clinical trial, participants received 160 mg selpercatinib orally twice daily until disease progression or unacceptable toxicity.[7] The major efficacy outcome measures were overall response rate (ORR), which reflects the percentage of participants that had a certain amount of tumor shrinkage, and duration of response (DOR).[7] The trial was conducted at 84 sites in the United States, Canada, Australia, Hong Kong, Japan, South Korea, Singapore, Taiwan, Switzerland, Germany, Denmark, Spain, France, the United Kingdom, Italy and Israel.[8]
Efficacy for non-small cell lung cancer (NSCLC) was evaluated in 105 adult participants with rearranged during transfection (RET) fusion-positive NSCLC who were previously treated with platinum chemotherapy.[7] Sixty-four percent of the 105 participants with previously treated NSCLC, experienced complete or partial shrinkage of their tumors which lasted more than six months for 81% of them.[8] Out of 39 participants who had never undergone treatment, 84% experienced complete or partial shrinkage of their tumors which lasted more than six months for 58% of them.[8]
Efficacy for medullary thyroid cancer (MTC) was evaluated in participants 12 years of age and older with RET-mutant MTC .[7] The study enrolled 143 participants with advanced or metastatic RET-mutant MTC who had been previously treated with cabozantinib, vandetanib or both (types of chemotherapy), and participants with advanced or metastatic RET-mutant MTC who had not received prior treatment with cabozantinib or vandetanib.[7] Sixty-nine percent of the 55 previously treated participants for MTC experienced complete or partial shrinkage of their tumors which lasted more than 6 months for 76% of them.[8] Out of 88 participants who had never undergone treatment with an approved drug, 73% experienced complete or partial shrinkage of their tumors which lasted more than six months for 61% of them.[8]
Efficacy for rearranged during transfection (RET) fusion-positive thyroid cancer was evaluated in participants 12 years of age and older.[7] The study enrolled 19 participants with RET fusion-positive thyroid cancer who were radioactive iodine-refractory (RAI, if an appropriate treatment option) and had received another prior systemic treatment, and eight participants with RET fusion-positive thyroid cancer who were RAI-refractory and had not received any additional therapy.[7] Seventy-nine percent of the 19 previously treated participants with thyroid cancer experienced complete or partial shrinkage of their tumors which lasted more than six months for 87% of them.[8] All eight participants who had not received therapy other than radioactive iodine therapy experienced complete or partial shrinkage of their tumors which lasted more than six months for 75% of them.[8]
The US Food and Drug Administration (FDA) granted the application for selpercatinib priority review, orphan drug, and breakthrough therapy designations;[7][11] and granted approval of Retevmo to Loxo Oncology, Inc., a subsidiary of Eli Lilly and Company.[7]
On 10 December 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Retsevmo, intended for the treatment of cancers that display rearranged during transfection (RET) gene alterations: RET-fusion positive non-small cell lung cancer (NSCLC), RET-fusion positive thyroid cancer and RET-mutant medullary-thyroid cancer (MTC).[12] The applicant for this medicinal product is Eli Lilly Nederland B.V. Selpercatinib was approved for medical use in the European Union in February 2021.[4]
References
- 1 2 3 4 "Selpercatinib Monograph for Professionals". Drugs.com. Archived from the original on 21 November 2021. Retrieved 12 October 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Retevmo- selpercatinib capsule". DailyMed. Archived from the original on 4 March 2021. Retrieved 31 March 2021.
- 1 2 3 "Retsevmo". Archived from the original on 23 April 2021. Retrieved 12 October 2021.
- 1 2 "Retsevmo EPAR". European Medicines Agency (EMA). 9 December 2020. Archived from the original on 23 April 2021. Retrieved 23 April 2021.
- 1 2 "Selpercatinib". SPS - Specialist Pharmacy Service. 14 February 2020. Archived from the original on 28 October 2020. Retrieved 12 October 2021.
- ↑ "Retevmo Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 12 October 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 "FDA Approves First Therapy for Patients with Lung and Thyroid Cancers with a Certain Genetic Mutation or Fusion". U.S. Food and Drug Administration (FDA) (Press release). 8 May 2020. Archived from the original on 9 May 2020. Retrieved 8 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 6 7 8 9 10 11 12 13 "Drug Trials Snapshots: Retevmo". U.S. Food and Drug Administration (FDA). 8 May 2020. Archived from the original on 20 October 2020. Retrieved 1 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Retevmo: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 21 October 2020. Retrieved 8 May 2020.
- 1 2 "Lilly Receives U.S. FDA Approval for Retevmo (selpercatinib), the First Therapy Specifically for Patients with Advanced RET-Driven Lung and Thyroid Cancers" (Press release). Eli Lilly and Company. 8 May 2020. Archived from the original on 9 May 2020. Retrieved 8 May 2020 – via PR Newswire.
- ↑ "FDA approves selpercatinib for lung and thyroid cancers with RET gene". U.S. Food and Drug Administration (FDA). 8 May 2020. Archived from the original on 12 May 2020. Retrieved 11 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Retsevmo: Pending EC decision". European Medicines Agency (EMA). 11 December 2020. Archived from the original on 11 December 2020. Retrieved 11 December 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Selpercatinib". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 3 June 2021. Retrieved 17 June 2021.
- "Selpercatinib". National Cancer Institute. 26 May 2020. Archived from the original on 9 April 2021. Retrieved 17 June 2021.
- "Understanding Metastatic RET Fusion-Positive Non-Small Cell Lung Cancer (NSCLC)" (PDF). Archived (PDF) from the original on 13 December 2020. Retrieved 17 June 2021.
- "Understanding Metastatic RET-Driven Thyroid Cancers" (PDF). Archived (PDF) from the original on 19 September 2020. Retrieved 17 June 2021.
- Li AY, McCusker MG, Russo A, et al. (December 2019). "RET fusions in solid tumors". Cancer Treat. Rev. 81: 101911. doi:10.1016/j.ctrv.2019.101911. PMID 31715421.
- Russo A, Lopes AR, McCusker MG, et al. (April 2020). "New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1)". Curr Oncol Rep. 22 (5): 48. doi:10.1007/s11912-020-00909-8. PMID 32296961. S2CID 215774750.