Radotinib
 | |
| Clinical data | |
|---|---|
| Trade names | Supect |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
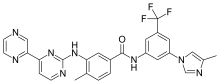
| Formula | C27H21F3N8O |
| Molar mass | 530.515 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Radotinib (INN; trade name Supect), and sometimes referred to by its investigational name IY5511, is a drug for the treatment of different types of cancer, most notably Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML)[1] with resistance or intolerance of other Bcr-Abl tyrosine-kinase inhibitors, such as patients resistant or intolerant to imatinib.
Radotinib is being developed by Ilyang Pharmaceutical Co., Ltd of South Korea[2] and is co-marketed by Daewoong Pharmaceutical Co. Ltd, in South Korea.[3] Radotinib completed a multi-national Phase II clinical trial study in 2012[4] and in August 2011, Ilyang initiated a Phase III, multinational, multi-center, open-label, randomized study for first-line indication.[5] Its mechanism of action involves inhibition of the Bcr-Abl tyrosine kinase and of platelet-derived growth factor receptor (PDGFR).[6]
References
- ↑ Joanne Bronson; Amelia Black; T. G. Murali Dhar; Bruce A. Ellsworth; J. Robert Merritt (2013). "To Market, To Market - 2012". Radotinib (Anticancer). Annual Reports in Medicinal Chemistry. Vol. 48. pp. 523–524. doi:10.1016/b978-0-12-417150-3.00028-4. ISBN 9780124171503.
- ↑ "Il-Yang Pharmaceutical".
- ↑ http://www.dailypharm.com/Users/News/EnglishNews.html?NewsID=3108&nStart=1023&mode=&searchValue=
- ↑ Kim SH, Menon H, Jootar S, Saikia T, Kwak JY, Sohn SK, Park JS, Jeong SH, Kim HJ, Kim YK, Oh SJ, Kim H, Zang DY, Chung JS, Shin HJ, Do YR, Kim JA, Kim DY, Choi CW, Park S, Park HL, Lee GY, Cho DJ, Shin JS, Kim DW (2014). "Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors". Haematologica. 99 (7): 1191–6. doi:10.3324/haematol.2013.096776. PMC 4077080. PMID 24705186.
- ↑ https://clinicaltrials.gov/ct2/show/NCT01511289?term=radotinib&rank=1
- ↑ "Radotinib hydrochloride". NCI Drug Dictionary. National Cancer Institute. 2011-02-02.