Tepotinib
 | |
 | |
| Names | |
|---|---|
| Trade names | Tepmetko |
| Other names | EMD-1214063 |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 450 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
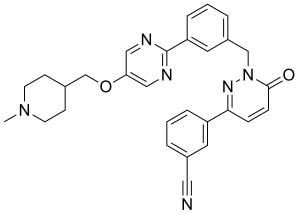
| Formula | C29H28N6O2 |
| Molar mass | 492.583 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tepotinib, sold under the brand name Tepmetko, is a medication used to treat non-small cell lung cancer (NSCLC).[2] Specifically it is used in metastatic disease that has mesenchymal-epithelial transition (MET) exon 14 skipping.[2] It is taken by mouth.[2]
Common side effects include swelling, tiredness, nausea, diarrhea, muscle pain, low sodium, and shortness of breath.[2] Other side effects may include pneumonitis and liver problems.[2] Use in pregnancy may harm the baby.[2] It is a kinase inhibitor that blocks MET.[2][7]
Tepotinib was approved in Japan in 2020, the United States in 2021, and Europe in 2022.[2][8][9] In the United Kingdom it costs the NHS about £7200 per month as of 2022.[9] This amount in the United States costs about 22,800 USD.[10]
Medical uses
Tepotinib is indicated for the treatment of adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping.[2][4] While a decrease in cancer was seen in 44% of 138 people, it was not studied against a control group.[7]
In Japan it was approved as a "line-agnostic" drug, meaning it is approved both for treatment-naive patients and for those in whom previous attempts at treatment have failed.[8] It is the second therapy in the USA, after capmatinib.[11]
Dosage
It is used at a dose of 450 mg once per day.[2]
Side effects
The most common side effects seen in clinical trials were edema, fatigue, nausea, diarrhea, muscle aches, and shortness of breath. Like capmatinib, tepotinib can also cause interstitial lung disease and liver damage, and is toxic to a developing fetus.[4] The most common treatment-related adverse effect in a 2021 study were peripheral edema (54.1%), nausea (20.0%), diarrhea (19.6%), blood creatinine increased (17.6%), and hypoalbuminemia (14.5%), which were 'mostly mild or moderate'.[12]
Society and culture
Legal status
On 16 December 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Tepmetko, intended for the treatment of patients with advanced non-small cell lung cancer (NSCLC) harboring alterations leading to mesenchymal-epithelial transition factor gene exon 14 (METex14) skipping.[13] The applicant for this medicinal product is Merck Europe B.V.[13] Tepotinib (Tepmetko) was approved for medical use in the European Union in February 2022.[5]
References
- 1 2 "Tepmetko APMDS". Therapeutic Goods Administration (TGA). 27 January 2022. Retrieved 5 February 2022.
{{cite web}}: CS1 maint: url-status (link) - 1 2 3 4 5 6 7 8 9 10 11 12 "Tepmetko- tepotinib hydrochloride tablet". DailyMed. Archived from the original on 27 November 2021. Retrieved 13 February 2021.
- ↑ "Summary Basis of Decision (SBD) for Tepmetko". Health Canada. Archived from the original on 29 May 2022. Retrieved 29 May 2022.
- 1 2 3 "FDA grants accelerated approval to tepotinib for metastatic non-small cell lung cancer". Food and Drug Administration. 3 February 2021. Archived from the original on 3 February 2021. Retrieved 3 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Tepmetko EPAR". European Medicines Agency (EMA). 14 December 2021. Archived from the original on 5 May 2022. Retrieved 5 May 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "FDA Approves Tepmetko as the First and Only Once-daily Oral MET Inhibitor for Patients with Metastatic NSCLC with METex14 Skipping Alterations". EMD Serono (Press release). 3 February 2021. Archived from the original on 4 February 2021. Retrieved 3 February 2021.
- 1 2 "Tepmetko". EMA. Archived from the original on 5 May 2022. Retrieved 31 October 2022.
- 1 2 "Tepmetko (Tepotinib) Approved in Japan for Advanced NSCLC with METex14 Skipping Alterations" (Press release). Merck KGaA. 25 March 2020. Retrieved 3 February 2021.
- 1 2 "Tepotinib". SPS - Specialist Pharmacy Service. 17 September 2019. Archived from the original on 21 January 2022. Retrieved 31 October 2022.
- ↑ "Tepmetko". Archived from the original on 31 October 2022. Retrieved 31 October 2022.
- ↑ Mathieu LN, Larkins E, Akinboro O, Roy P, Amatya AK, Fiero MH, et al. (January 2022). "FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations". Clin Cancer Res. 28 (2): 249–254. doi:10.1158/1078-0432.CCR-21-1566. PMID 34344795. S2CID 236915283.
- ↑ Morise, Masahiro; Sakai, Hiroshi; Veillon, Remi; Le, Xiuning; Felip, Enriqueta; Garassino, Marina Chiara; Cortot, Alexis B.; Smit, Egbert; Park, Keunchil; Griesinger, Frank; Britschgi, Christian (July 2021). "O13-4 Tepotinib safety in MET exon 14 (METex14) skipping NSCLC: Updated results from the VISION trial". Annals of Oncology. 32: S291. doi:10.1016/j.annonc.2021.05.541. ISSN 0923-7534. S2CID 237785148. Archived from the original on 30 September 2022. Retrieved 4 October 2021.
- 1 2 "Tepmetko: Pending EC decision". European Medicines Agency. 17 December 2021. Archived from the original on 18 December 2021. Retrieved 18 December 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. (September 2020). "Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations". N Engl J Med. 383 (10): 931–43. doi:10.1056/NEJMoa2004407. PMC 8422679. PMID 32469185.
External links
| Identifiers: |
|---|
- "Tepotinib". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 October 2022. Retrieved 30 September 2022.
- "Tepotinib hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
- "Tepotinib hydrochloride". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 10 August 2019. Retrieved 30 September 2022.
- Clinical trial number NCT02864992 for "Tepotinib Phase II in Non-small Cell Lung Cancer (NSCLC) Harboring MET Alterations (VISION)" at ClinicalTrials.gov