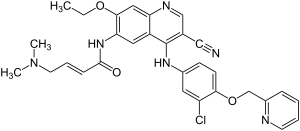
Neratinib
 | |
| Names | |
|---|---|
| Trade names | Nerlynx, Hernix |
| Other names | HKI-272 |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Breast cancer[2] |
| Side effects | Diarrhea, nausea, tiredness, belly pain, rash, inflamed mouth, muscle spasms[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617034 |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C30H29ClN6O3 |
| Molar mass | 557.05 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Neratinib, sold under the brand name Nerlynx, is a medication used to treat breast cancer.[2][3] Specifically it is used for HER2 positive and hormone-receptor positive cases following surgery and trastuzumab.[3] It is taken by mouth.[1]
Diarrhea affects nearly all people.[3] Other common side effects include nausea, tiredness, belly pain, rash, inflamed mouth, and muscle spasms.[3] Other side effects may include liver problems.[1] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor which blocks HER2, HER4, and EGFR.[1]
Neratinib was approved for medical use in the United States in 2017 and Europe in 2018.[1][3] In the United States it costs 19,200 USD per month as of 2021.[5] In the United Kingdom this amount costs about £4,500.[6]
Medical uses
In the European Union and the United States, neratinib is indicated for the extended adjuvant treatment of adults with early stage hormone receptor positive HER2-overexpressed/amplified breast cancer and who are less than one year from the completion of prior adjuvant trastuzumab based therapy.[2][3]
In the United States, it is also indicated, in combination with capecitabine, for the treatment of adults with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting.[2]
Dosage
It is generally take at a dose of 240 mg per day.[1] It is taken for 1 year.[6]
In those taking a strong CYP3A4 inhibitors a dose of 40 mg per day is recommended.[6]
Contraindications
Women who are pregnant should not take it, and women should not become pregnant while taking it, and women who are breast-feeding should not use it, as it can cause harm to the fetus and to the baby.[2]
Side effects
Neratinib can cause life-threatening diarrhea in some people and mild to moderate diarrhea in almost everyone; people who take it are also at risk for complications of diarrhea like dehydration and electrolyte imbalance.[2] Similarly, there is a risk of severe liver damage and many patients have some level of it; symptoms of liver damage include fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, rash, and high levels of eosinophils.[2]
In addition to the above, more than 10% of people taking it have nausea, abdominal pain, vomiting, sores on their lips, stomach upset, decreased appetite, rashes, and muscle spasms.[2]
Interactions
People taking neratinib should not also take gastric acid reducing agents including proton pump inhibitors and H2-receptor antagonists; antacids may be used three hours before of after taking it.[2]
Drugs that inhibit CYP3A4 increase the activity of neratinib and can make adverse effects worse, and drugs that induce CYP3A4 make neratinib less active and can reduce its efficacy. Neratinib also inhibits p-glycoprotein and effectively raises the dose of drugs like digoxin that depend on it for elimination.[2]
Pharmacology
Like lapatinib and afatinib, it is a dual inhibitor of the human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) kinases.[7][8] It inhibits them by covalently binding with a cysteine side chain in those proteins.[9] Unlike related noncovalent inhibitors, neratinib is effective against the T790M resistant variant of EGFR.[10]
Neratinib has an IC50 of 59 nM against HER2 and shows weak inhibition against KDR and Scr with IC50 values of 0.8 μM and 1.4 μM, respectively. In BT474 cells, neratinib reduces HER2 autophosphorylation, and inhibited cyclin D1 expression while reduced proliferation has been observed A431 cells when treated with neratinib at concentrations of 3 or 5 nM. In xenograft models with 3T3/neu tumors oral administration of neratinib at 10, 20, 40 or 80 mg/kg was able to inhibit tumor growth while in SK-OV-3 models doses of 5 and 60 mg/kg significantly inhibited tumor growth.
Cell biology
Neratinib is found to strongly reduce the amount of HER2 released by extracellular vescicles and to enhance the capacity of clathrin mediated endocytosis. However, despite HER2 mediated signaling downregulation, Neratinib exerts only a modest effect on HER2 trafficking at IC50 of 6nM in SKBR3 cells. [11]
Chemistry
Neratinib is a 4-anilino-3-cyano quinoline derivative.[2]
History
Neratinib was discovered and initially developed by Wyeth; Pfizer continued development up to Phase III in breast cancer, and licensed it to Puma Biotechnology in 2011.[12]
It was approved for medical use in the United States in July 2017, for the extended adjuvant treatment of adults with early stage HER2-overexpressed/amplified breast cancer, (after adjuvant trastuzumab-based therapy).[13][14][15] Approval was based on the ExteNET trial (NCT00878709), a multicenter, randomized, double-blind, placebo-controlled trial of neratinib following adjuvant trastuzumab treatment.[14][15][16]
Neratinib was approved for medical use in the European Union in August 2018.[3]
Society and culture
Brand names
In Bangladesh it is sold under the trade name Hernix.[17]
References
- 1 2 3 4 5 6 7 "Neratinib Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 12 November 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Nerlynx- neratinib tablet". DailyMed. 6 August 2020. Archived from the original on 18 November 2020. Retrieved 13 November 2020.
- 1 2 3 4 5 6 7 8 9 "Nerlynx EPAR". European Medicines Agency (EMA). Archived from the original on 20 November 2020. Retrieved 13 November 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Nerlynx 40 mg Film-coated Tablets - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 28 October 2021. Retrieved 13 November 2020.
- ↑ "Nerlynx Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 21 April 2021. Retrieved 12 November 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1035. ISBN 978-0857114105.
- ↑ Baselga J, Coleman RE, Cortés J, Janni W (November 2017). "Advances in the management of HER2-positive early breast cancer". Critical Reviews in Oncology/Hematology. 119: 113–122. doi:10.1016/j.critrevonc.2017.10.001. PMC 5662944. PMID 29042085.
- ↑ Minami Y, Shimamura T, Shah K, LaFramboise T, Glatt KA, Liniker E, et al. (July 2007). "The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272". Oncogene. 26 (34): 5023–7. doi:10.1038/sj.onc.1210292. PMID 17311002.
- ↑ Singh J, Petter RC, Baillie TA, Whitty A (April 2011). "The resurgence of covalent drugs". Nature Reviews. Drug Discovery. 10 (4): 307–17. doi:10.1038/nrd3410. PMID 21455239. S2CID 5819338.
- ↑ Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. (12 February 2008). "The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP". Proceedings of the National Academy of Sciences of the United States of America. 105 (6): 2070–5. Bibcode:2008PNAS..105.2070Y. doi:10.1073/pnas.0709662105. PMC 2538882. PMID 18227510.
- ↑ Santamaria, S; Gagliani, MC; Bellese, G; Marconi, S; Aiello, C; Tagliatti, E; Castagnola, P; Cortese, K (2021). "Imaging of Endocytic Trafficking and Extracellular Vesicles Released Under Neratinib Treatment in ERBB2 + Breast Cancer Cells". J Histochem Cytochem. 69 (7): 461–473. doi:10.1369/00221554211026297. PMC 8246527. PMID 34126793.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - ↑ "Puma Acquires Global Rights to Pfizer's Phase III Breast Cancer Drug Neratinib". GEN. 6 October 2011. Archived from the original on 20 September 2018. Retrieved 11 September 2021.
- ↑ "Nerlynx (neratinib maleate) Tablets". U.S. Food and Drug Administration (FDA). 21 August 2017. Archived from the original on 14 November 2020. Retrieved 13 November 2020.
- 1 2 "FDA approves new treatment to reduce the risk of breast cancer returning". U.S. Food and Drug Administration (FDA) (Press release). 17 July 2017. Archived from the original on 27 October 2020. Retrieved 13 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "FDA approves neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer". U.S. Food and Drug Administration (FDA). 17 July 2017. Archived from the original on 29 October 2020. Retrieved 13 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Drug Trials Snapshot: Nerlynx". U.S. Food and Drug Administration (FDA). 17 July 2017. Archived from the original on 2 December 2020. Retrieved 13 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Hernix". medex.com.bd. Archived from the original on 16 July 2021. Retrieved 14 July 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Neratinib maleate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 16 November 2020. Retrieved 11 September 2021.
- "Neratinib maleate". National Cancer Institute. 27 July 2017. Archived from the original on 6 October 2021. Retrieved 11 September 2021.
- "Neratinib maleate". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 17 January 2021. Retrieved 11 September 2021.