Pemigatinib
 | |
| Names | |
|---|---|
| Trade names | Pemazyre |
| Other names | INCB054828 |
IUPAC name
| |
| Clinical data | |
| Drug class | Kinase inhibitor[1] |
| Main uses | Bile duct cancer[1][2] |
| Side effects | High or low phosphate, hair loss, diarrhea, tiredness, taste disturbances, inflammation of the mouth, dry eyes, rash, kidney problems[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 13.5 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620028 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
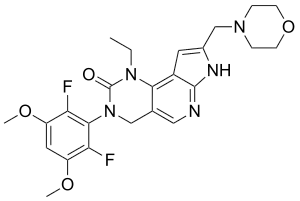
| Formula | C24H27F2N5O4 |
| Molar mass | 487.508 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pemigatinib, sold under the brand name Pemazyre, is a medication used to treat bile duct cancer (cholangiocarcinoma).[1][2] It is used in cases which are FGFR2 positive and have failed other treatments.[2][3] It is taken by mouth.[2]
Common side effects include high or low phosphate, hair loss, diarrhea, tiredness, taste disturbances, inflammation of the mouth, dry eyes, rash, and kidney problems.[2] Other side effects may include eye problems.[1] Use in pregnancy may harm the baby.[1] It is a kinase inhibitor and works by blocking fibroblast growth factor receptors.[2][1]
Pemigatinib was approved for medical use in the United States in 2020.[1] It received conditional approval in Europe in 2021.[2] In the United States it costs about 17,800 USD every three weeks.[4] In the United Kingdom this amount costs the NHS about £7200.[5]
Medical uses
Pemigatinib is indicated for the treatment of adults with locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or rearrangement that have progressed after at least one prior line of systemic therapy.[1][2][6]
Cholangiocarcinoma is a rare form of cancer that forms in bile ducts, which are slender tubes that carry the digestive fluid bile from the liver to gallbladder and small intestine.[6] Pemigatinib is indicated for the treatment of adults with bile duct cancer (cholangiocarcinoma) that is locally advanced (when cancer has grown outside the organ it started in, but has not yet spread to distant parts of the body) or metastatic (when cancer cells spread to other parts of the body) and who have tumors that have a fusion or other rearrangement of a gene called fibroblast growth factor receptor 2 (FGFR2).[6] It should be used in patients who have been previously treated with chemotherapy and whose cancer has a certain type of abnormality in the FGFR2 gene.[7]
Dosage
It is generally take at a dose of 13.5 mg per day for 14 days followed by seven days of no treatment.[1] This is than repeated.[1] Doses may be adjusted based on side effects.[3]
History
Pemigatinib was approved for use in the United States in April 2020 along with the FoundationOne CDX (Foundation Medicine, Inc.) as a companion diagnostic for patient selection.[6][8] [9]
The approval of pemigatinib in the United States was based on the results the FIGHT-202 (NCT02924376) multicenter open-label single-arm trial that enrolled 107 participants with locally advanced or metastatic cholangiocarcinoma with an FGFR2 fusion or rearrangement who had received prior treatment.[6][8][7] The trial was conducted at 67 sites in the United States, Europe, and Asia.[7] During the clinical trial, participants received pemigatinib once a day for 14 consecutive days, followed by 7 days off, in 21-day cycles until the disease progressed or the patient experienced an unreasonable level of side effects.[6][8][7] To assess how well pemigatinib was working during the trial, participants were scanned every eight weeks.[6] The trial used established criteria to measure how many participants experienced a complete or partial shrinkage of their tumors during treatment (overall response rate).[6] The overall response rate was 36% (95% CI: 27%, 45%), with 2.8% of participants having a complete response and 33% having a partial response.[6] Among the 38 participants who had a response, 24 participants (63%) had a response lasting six months or longer and seven participants (18%) had a response lasting 12 months or longer.[6][8]
The U.S. Food and Drug Administration (FDA) granted the application for pemigatinib priority review, breakthrough therapy and orphan drug designations.[6][8][10][11] The FDA granted approval of Pemazyre to Incyte Corporation.[6]
On 24 August 2018, orphan designation (EU/3/18/2066) was granted by the European Commission to Incyte Biosciences Distribution B.V., the Netherlands, for pemigatinib for the treatment of biliary tract cancer.[12] On 17 October 2019, orphan designation EU/3/19/2216 was granted by the European Commission to Incyte Biosciences Distribution B.V., the Netherlands, for pemigatinib for the treatment of myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, or FGFR1, or with PCM1-JAK2.[13] On 28 January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Pemazyre, intended for the second-line treatment of advanced or metastatic cholangiocarcinoma characterized by fusion or rearrangements of fibroblast growth factor receptor 2.[14] The applicant for this medicinal product is Incyte Biosciences Distribution B.V.[14] Pemigatinib was approved for medical use in the European Union in March 2021.[2]
Names
Pemigatinib is the international nonproprietary name (INN).[15]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Pemazyre- pemigatinib tablet". DailyMed. Archived from the original on 6 February 2021. Retrieved 1 February 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Pemazyre EPAR". European Medicines Agency (EMA). 25 January 2021. Archived from the original on 2 September 2021. Retrieved 1 September 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2
- ↑ "Pemazyre Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 27 October 2021.
- ↑ "Pemigatinib". SPS - Specialist Pharmacy Service. 11 June 2019. Archived from the original on 27 October 2021. Retrieved 27 October 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 "FDA Approves First Targeted Treatment for Patients with Cholangiocarcinoma, a Cancer of Bile Ducts". U.S. Food and Drug Administration (FDA) (Press release). 17 April 2020. Archived from the original on 18 April 2020. Retrieved 17 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 "Drug Trials Snapshot: Pemazyre". U.S. Food and Drug Administration (FDA). 17 April 2020. Archived from the original on 4 August 2020. Retrieved 5 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 "FDA grants accelerated approval to pemigatinib for cholangiocarcinoma". U.S. Food and Drug Administration (FDA). 17 April 2020. Archived from the original on 21 April 2020. Retrieved 20 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Pemazyre: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 19 September 2020. Retrieved 21 April 2020.
- ↑ "Pemigatinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Archived from the original on 28 February 2021. Retrieved 19 April 2020.
- ↑ "Pemigatinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Archived from the original on 28 February 2021. Retrieved 19 April 2020.
- ↑ "EU/3/18/2066". European Medicines Agency (EMA). 19 December 2018. Archived from the original on 24 October 2020. Retrieved 20 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "EU/3/19/2216". European Medicines Agency (EMA). 23 January 2020. Archived from the original on 28 November 2020. Retrieved 19 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Pemazyre: Pending EC decision". European Medicines Agency (EMA). 29 January 2021. Archived from the original on 1 February 2021. Retrieved 2 February 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80". WHO Drug Information. 32 (3): 479. hdl:10665/330907.
Further reading
- Roskoski R (January 2020). "The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder". Pharmacol. Res. 151: 104567. doi:10.1016/j.phrs.2019.104567. PMID 31770593.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Pemigatinib". National Cancer Institute. Archived from the original on 14 July 2020. Retrieved 2 September 2021.
- Clinical trial number NCT02924376 for "Efficacy and Safety of Pemigatinib in Subjects With Advanced/Metastatic or Surgically Unresectable Cholangiocarcinoma Who Failed Previous Therapy - (FIGHT-202)" at ClinicalTrials.gov