Vandetanib
 | |
| Names | |
|---|---|
| Trade names | Caprelsa |
| Other names | ZD6474 |
IUPAC name
| |
| Clinical data | |
| Drug class | Kinase inhibitor[1] |
| Main uses | Medullary thyroid cancer[1] |
| Side effects | QT prolongation, sun sensitivity, acne, high blood pressure, headache, low calcium[1][2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth[1] |
| Typical dose | 300 mg/day[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611037 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 90–96% |
| Metabolism | CYP3A4, FMO1, FMO3 |
| Elimination half-life | 19 days (mean)[3] |
| Excretion | 44% faeces, 25% urine |
| Chemical and physical data | |
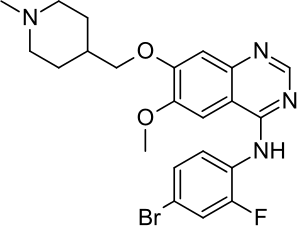
| Formula | C22H24BrFN4O2 |
| Molar mass | 475.362 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vandetanib, sold under the brand name Caprelsa, is an anti-cancer medication used to treat a type of thyroid cancer, specifically medullary thyroid cancer.[1] It increases the amount of time before the disease worsens.[1] It is taken by mouth.[1]
Common side effects include acne, high blood pressure, headache, low calcium, low blood sugar, and diarrhea.[1] Other side effects include QT prolongation, sun sensitivity, hair loss, swelling, and corneal deposits.[1][2] Use during pregnancy or breastfeeding may harm the baby.[4] It works as a kinase inhibitor, mainly of the vascular endothelial growth factor receptor 2 (VEGFR2) and the epidermal growth factor receptor (EGFR).[5]
Vandetanib was approved for medical use in the United States in 2011.[1] In the United Kingdom it costs the NHS £5,000 per month as of 2020.[2] In the United States this amount costs about 16,000 USD as of 2021;[6] while in Canada it cost about 5,900 CAD per month as of 2017.[7]
Medical use
Vandetanib is used to treat medullary thyroid cancer in adults who are ineligible for surgery.[3][8][9]
Dosage
It is generally taken at a dose of 300 mg per day though can be used at a lower dose in those with too many side effects.[1] It is taken long term.[1]
Contraindications
The V804M mutation in RET confers resistance to vandetanib anti-RET activity.[9]
In people with moderate and severe hepatic impairment, no dosage for vandetanib has been recommended, as its safety and efficacy has not been established yet.[10] Vandetanib is contraindicated in people with congenital long QT syndrome.[3][11]
Side effects
Very common (present in greater than 10% of people) adverse effects include colds, bronchitis, upper respiratory tract infections, urinary tract infections, decreased appetite, low calcium absorption, insomnia, depressed mood, Headache, tingling sensations, weird, painful sensations, dizziness, blurred vision, damage to the cornea, long QT syndrome, high blood pressure, stomach pain, diarrhea, nausea, vomiting, indigestion, sensitivity to sunlight, rash, acne, dry and itchy skin, nail disorders, protein in urine, kidney stones, weakness, fatigue, pain, and edema.[8]
Common (present in between 1% and 10% of people) adverse effects include pneumonia, sepsis, influenza, cystitis, sinusitis, laryngitis, folliculitis, boils, fungal infection, kidney infections, low thyroid hormone levels, low potassium, high calcium levels, hyperglycemia, dehydration, low sodium levels, anxiety, tremor, lethargy, loss of consciousness, balance disorders, changes in sense of taste, visual impairment, halo vision, perceived light flashes, glaucoma, pink eye, dry eye, keratopathy, hypertensive crisis, mini strokes, nose bleeds, coughing up blood, defecating blood, colitis, dry mouth, stomatitis, constipation, gastritis, gallstones, Chemotherapy-induced acral erythema, hair loss, painful urination, bloody urine, kidney failure, frequent urination, urgent need to urinate, and fever.[8]
Interactions
Vandetanib has been reported as a substrate for the OATP1B1 and OATP1B3 transporters. Interaction of vandetanib with OATP1B1 and OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions.[10] Also, vandetanib is an inhibitor of OATP1B3 transporter but not for OATP1B1.[12]
Other drugs that prolong the QT interval can possibly add to this side effect of vandetanib. As the drug is partly metabolised via the liver enzyme CYP3A4, strong inducers of this enzyme can decrease its blood plasma concentrations. CYP3A4 inhibitors do not significantly increase vandetanib concentrations, presumably because it is also metabolised by flavin containing monooxygenase 1 (FMO1) and 3.[3][11]
Pharmacology

Vandetanib is an inhibitor of vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and RET tyrosine kinases. RET tyrosine kinases; it weakly inhibits VEGFR-3.[8][14]
Vandetanib is well absorbed from the gut, reaches peak blood plasma concentrations 4 to 10 hours after application, and has a half-life of 19 days on average, per pharmacokinetic studies. It has to be taken for about three months to achieve a steady-state concentration. In the blood, it is almost completely (90–96%) bound to plasma proteins such as albumin. It is metabolised to N-desmethylvandetanib via CYP3A4 and to vandetanib-N-oxide via FMO1 and 3. Both of these are active metabolites. Vandetanib is excreted via the faeces (44%) and the urine (25%) in form of the unchanged drug and the metabolites.[11][15][13]
History
Vandetanib was approved by the FDA in April 2011, for treatment of late-stage thyroid cancer.[16]
Vandetanib was first initially marketed without a trade name; it has been marketed under the trade name Caprelsa since August 2011.[17]
The drug was developed by AstraZeneca[3] who later sold the rights to Sanofi in 2015.[18][19] In 2015 Genzyme acquired the product from AstraZeneca.[20]
Research
AstraZeneca tested Vandetanib in clinical trials for non-small cell lung cancer and submitted an application for approval to the EMA but then withdrew the application in October 2009 after trials showed no benefit when the drug was administered alongside chemotherapy.[21] A clinical trial of vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma was negative in a prospective, randomised, double-blind, multicentre phase 2 trial.[22]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Vandetanib Monograph for Professionals". Drugs.com. Archived from the original on 24 July 2012. Retrieved 6 August 2021.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1061. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 3 4 5 6 "Caprelsa- vandetanib tablet, film coated". DailyMed. 19 June 2020. Archived from the original on 27 October 2020. Retrieved 8 December 2020.
- ↑ "Vandetanib (Caprelsa) Use During Pregnancy". Drugs.com. Archived from the original on 29 October 2020. Retrieved 6 August 2021.
- ↑ "Definition of vandetanib". NCI Drug Dictionary. National Cancer Institute. 2011-02-02. Archived from the original on 2018-01-24. Retrieved 2021-05-02.
- ↑ "Caprelsa Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 27 August 2021. Retrieved 6 August 2021.
- ↑ "pCODR Expert Review Committee Final Recommendation" (PDF). CADTH. Archived (PDF) from the original on 19 October 2018. Retrieved 6 August 2021.
- 1 2 3 4 "UK label". www.medicines.org.uk. UK Electronic Medicines Compendium. 16 December 2016. Archived from the original on 28 February 2017. Retrieved 27 February 2017.
- 1 2 Viola, D; et al. (April 2016). "Treatment of advanced thyroid cancer with targeted therapies: ten years of experience". Endocrine-Related Cancer. 23 (4): R185–205. doi:10.1530/ERC-15-0555. PMID 27207700.
- 1 2 Khurana V, Minocha M, Pal D, Mitra AK (March 2014). "Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors". Drug Metabol Drug Interact. 0 (3): 179–90. doi:10.1515/dmdi-2013-0062. PMC 4407685. PMID 24643910.
- 1 2 3 "Vandetanib Monograph". Drugs.com. Archived from the original on 24 July 2012. Retrieved 29 August 2012.
- ↑ Khurana V, Minocha M, Pal D, Mitra AK (May 2014). "Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors". Drug Metabol Drug Interact. 0 (4): 249–59. doi:10.1515/dmdi-2014-0014. PMC 4407688. PMID 24807167.
- 1 2 "Clinical Pharmacology Review: Vandetanib" (PDF). US Food and Drug Administration, Center for Drug Evaluation and Research. 20 August 2010. Archived (PDF) from the original on 14 July 2014. Retrieved 29 August 2012.
- ↑ Carlomagno, F; Vitagliano, D; Guida, T; Ciardiello, F; Tortora, G; Vecchio, G; Ryan, AJ; Fontanini, G; Fusco, A; Santoro, M (15 December 2002). "ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases". Cancer Research. 62 (24): 7284–90. PMID 12499271. Archived from the original on 26 July 2019. Retrieved 2 May 2021.
- ↑ Martin, P.; Oliver, S.; Kennedy, S. J.; Partridge, E.; Hutchison, M.; Clarke, D.; Giles, P. (2012). "Pharmacokinetics of Vandetanib: Three Phase I Studies in Healthy Subjects". Clinical Therapeutics. 34 (1): 221–237. doi:10.1016/j.clinthera.2011.11.011. PMID 22206795.
- ↑ "FDA approves new treatment for rare form of thyroid cancer". Archived from the original on 10 April 2011. Retrieved 7 April 2011.
- ↑ Starkey, Jonathan (August 2, 2011). "AstraZeneca (finally) lands name for cancer drug". Delaware Inc. Archived from the original on August 27, 2021. Retrieved May 2, 2021.
- ↑ "AZ sells rare cancer drug to Sanofi". PMLive. 2015-07-27. Archived from the original on 2021-02-06. Retrieved 2021-01-26.
- ↑ "Genzyme to Buy Caprelsa from AstraZeneca for Up to $300M". GEN - Genetic Engineering and Biotechnology News. 2015-07-27. Archived from the original on 2021-01-30. Retrieved 2021-01-26.
- ↑ Fourcade, Marthe (27 July 2015). "Sanofi to Buy Caprelsa Drug from AstraZeneca for $300 Million". Bloomberg. Archived from the original on 2017-02-28. Retrieved 2021-05-02.
- ↑ "Zactima". European Medicines Agency. Archived from the original on 2018-09-20. Retrieved 2021-11-10.
- ↑ Middleton, Gary; Palmer, Daniel H; Greenhalf, William; Ghaneh, Paula; Jackson, Richard; Cox, Trevor; Evans, Anthony; Shaw, Victoria E; Wadsley, Jonathan; Valle, Juan W; Propper, David; Wasan, Harpreet; Falk, Stephen; Cunningham, David; Coxon, Fareeda; Ross, Paul; Madhusudan, Srinivasan; Wadd, Nick; Corrie, Pippa; Hickish, Tamas; Costello, Eithne; Campbell, Fiona; Rawcliffe, Charlotte; Neoptolemos, John P (2017). "Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial" (PDF). The Lancet Oncology. 18 (4): 486–499. doi:10.1016/S1470-2045(17)30084-0. PMID 28259610. Archived (PDF) from the original on 2021-03-06. Retrieved 2021-05-02.
External links
| External sites: |
|
|---|---|
| Identifiers: |