Lapatinib
 | |
| Names | |
|---|---|
| Trade names | Tykerb, Tyverb, others |
| Other names | Lapatinib ditosylate |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607055 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Variable, increased with food |
| Protein binding | >99% |
| Metabolism | Liver, mostly CYP3A-mediated (minor 2C19 and 2C8 involvement) |
| Elimination half-life | 24 hours (repeated dosing), 14.2 hours (single dose) |
| Excretion | Mostly Feces |
| Chemical and physical data | |
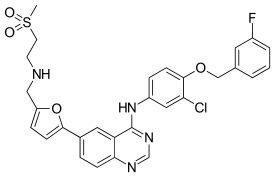
| Formula | C29H26ClFN4O4S |
| Molar mass | 581.06 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Lapatinib, sold under the brand names Tykerb and Tyverb is a medication used to treat breast cancer.[1] Specifically it is used for HER2 positive breast cancer with either letrozole, trastuzumab, or capecitabine.[1][2] It is taken by mouth.[1]
Common side effects include diarrhea, nausea, rash, tiredness, liver problems, itchiness, and hair loss.[1] Other side effects may include QT prolongation, pneumonitis, low platelets, low red blood cells, or low white blood cells.[1] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor which blocks HER2/neu and epidermal growth factor receptor (EGFR).[3]
Lapatinib was approved for medical use in the United States in 2007 and Europe in 2008.[1][2] In the United Kingdom 4 weeks of 1,000 mg costs the NHS about £970 as of 2021.[4] This amount in the United States costs about 4,300 USD.[5]
Medical uses
Breast cancer
Lapatinib is used as a treatment for women's breast cancer in treatment-naïve, ER+/EGFR+/HER2+ breast cancer patients and in patients who have HER2-positive advanced breast cancer that has progressed after previous treatment with other chemotherapeutic agents, such as anthracycline, taxane-derived drugs, or trastuzumab (Herceptin).
A 2006 GSK-supported trial on female breast cancer previously being treated with those agents (anthracycline, a taxane and trastuzumab) demonstrated that administrating lapatinib in combination with capecitabine delayed the time of further cancer growth compared to regimens that use capecitabine alone. The study also reported that risk of disease progression was reduced by 51%, and that the combination therapy was not associated with increases in toxic side effects.[6] The outcome of this study resulted in a somewhat complex and rather specific initial indication for lapatinib—use only in combination with capecitabine for HER2-positive breast cancer in women whose cancer have progressed following previous chemotherapy with anthracycline, taxanes and trastuzumab.
Dosage
It is generally used at a dose of 1,000 mg to 1,500 mg per day.[2]
Side effects
Like many small molecule tyrosine kinase inhibitors, lapatinib is regarded as well tolerated. The most common side effects reported are diarrhea, fatigue, nausea and rashes.[3][7] Of note, lapatinib related rash is associated with improved outcome.[8] In clinical studies elevated liver enzymes have been reported. QT prolongation has been observed with the use of lapatinib ditosylate but there are no reports of torsades de pointes. Caution is advised in patients with hypokalaemia, hypomagnesaemia, congenital long QT syndrome, or with coadministration of medicines known to cause QT prolongation. In combination with capecitabine, reversible decreased left ventricular function are common (2%).[9]
Mechanism of action
Biochemistry
Lapatinib inhibits the tyrosine kinase activity associated with two oncogenes, EGFR (epidermal growth factor receptor) and HER2/neu (human EGFR type 2).[10] Over expression of HER2/neu can be responsible for certain types of high-risk breast cancers in women.[3]
Like sorafenib, lapatinib is a protein kinase inhibitor shown to decrease tumor-causing breast cancer stem cells.[11]
Lapatinib inhibits receptor signal processes by binding to the ATP-binding pocket of the EGFR/HER2 protein kinase domain, preventing self-phosphorylation and subsequent activation of the signal mechanism (see Receptor tyrosine kinase#Signal transduction).[12]
History
On March 13, 2007, the U.S. Food and Drug Administration (FDA) approved lapatinib in combination therapy for breast cancer patients already using capecitabine (Xeloda).[3][13] In January 2010, Tykerb received accelerated approval for the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpresses the HER2 receptor and for whom hormonal therapy is indicated (in combination with letrozole).[13]
Pharmaceutical company GlaxoSmithKline (GSK) markets the drug under the propriety names Tykerb (mostly U.S.) and Tyverb (mostly Europe and Russia).[14] The drug currently has approval for sale and clinical use in the US,[3][14] Australia,[3] Bahrain,[3] Kuwait,[3] Venezuela,[3] Brazil,[15] New Zealand,[15] South Korea,[15] Switzerland,[14] Japan, Jordan, the European Union, Lebanon, India and Pakistan.[14]
On August 2, 2013, India's Intellectual Property Appellate Board revoked the patent for Glaxo's Tykerb citing its derivative status, while upholding at the same time the original patent granted for lapatinib.[16]
The drug lapatinib ditosylate is classified as S/NM (a synthetic compound showing competitive inhibition of the natural product) that is naturally derived or inspired substrate [17]
Research
Phase III study designed to assess lapatinib in combination with chemotherapy for advanced HER2-positive gastric cancer in 2013 failed to meet the primary endpoint of improved overall survival (OS) against chemotherapy alone. The trial did not discover new safety signals, while the median OS for patients in the lapatinib and chemotherapy group was 12.2 months against 10.5 months for patients in the placebo plus chemotherapy. Secondary endpoints of the randomized, double-blinded study, were progression-free survival (PFS), response rate and duration of response. Median PFS was 6 months, response rate was 53% and the duration of response was 7.3 months in the investigational combination chemotherapy group compared to median PFS of 5.4 months, response rate of 39% and duration of response of 5.6 months for patients in chemotherapy alone group. Diarrhoea, vomiting, anemia, dehydration and nausea were serious adverse events (SAE) reported in over 2% of patients in the investigational combination chemotherapy group, while vomiting was the most common SAE noted in the chemotherapy group.[18]
References
- 1 2 3 4 5 6 7 "Lapatinib Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 20 November 2021.
- 1 2 3 "Tyverb". Archived from the original on 23 October 2021. Retrieved 20 November 2021.
- 1 2 3 4 5 6 7 8 9 Higa GM, Abraham J (September 2007). "Lapatinib in the treatment of breast cancer". Expert Review of Anticancer Therapy. 7 (9): 1183–92. doi:10.1586/14737140.7.9.1183. PMID 17892419. S2CID 36837880. Archived from the original on 2021-05-25. Retrieved 2021-10-03.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1031. ISBN 978-0857114105.
- ↑ "Lapatinib Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 20 November 2021.
- ↑ Geyer CE, Forster J, Lindquist D, et al. (December 2006). "Lapatinib plus capecitabine for HER2-positive advanced breast cancer". N. Engl. J. Med. 355 (26): 2733–43. doi:10.1056/NEJMoa064320. PMID 17192538.
- ↑ Burris HA, Hurwitz HI, Dees EC, et al. (August 2005). "Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas". J. Clin. Oncol. 23 (23): 5305–13. doi:10.1200/JCO.2005.16.584. PMID 15955900.
- ↑ Sonnenblick A et al. Lapatinib-Related Rash and Breast Cancer Outcome in the ALTTO Phase III Randomized Trial.J Natl Cancer Inst. 2016 Apr
- ↑ NCI Cancer Drug Information. FDA Approval for Lapatinib Ditosylate (Tykerb®) Archived 2015-04-06 at the Wayback Machine. Retrieved 27 January 2014.
- ↑ Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, et al. (2004). "A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells". Cancer Research. 64 (18): 6652–9. doi:10.1158/0008-5472.CAN-04-1168. PMID 15374980.
- ↑ Angel Rodriguez (April 2008). New type of drug shrinks primary breast cancer tumors significantly in just six weeks; research provides leads to a new target in cancer treatment – the cancer stem cell. Archived from the original on 2008-11-26.
- ↑ Nelson MH, Dolder CR (February 2006). "Lapatinib: a novel dual tyrosine kinase inhibitor with activity in solid tumors". Ann Pharmacother. 40 (2): 261–9. doi:10.1345/aph.1G387. PMID 16418322. S2CID 21622641.
- 1 2 Pazdur, Richard (14 January 2011). "FDA Approval for Lapatinib Ditosylate". Womens Health (Lond Engl). Cancer.gov. 6 (2): 173. doi:10.2217/whe.10.11. PMID 20187722.
- 1 2 3 4 "GlaxoSmithKline receives marketing authorisation in the EU for Tyverb (lapatinib), the first oral targeted therapy for ErbB2-positive breast cancer" (Press release). GlaxoSmithKline. 2008-06-12. Archived from the original on 2008-06-15. Retrieved 2008-06-21.
- 1 2 3 "GlaxoSmithKline Reports Positive New Data On Tykerb (lapatinib) At The 2007 American Society Of Clinical Oncology (ASCO) Annual Meeting" (Press release). Medical News Today. June 4, 2007. Archived from the original on April 13, 2010. Retrieved October 3, 2021. Retrieved December 2, 2008.
- ↑ Kulkarni, Kaustubh (2 August 2013). "India revokes GSK cancer drug patent in latest Big Pharma blow". Reuters. Mumbai, India. Archived from the original on 7 March 2016. Retrieved 2 August 2013.
- ↑ (Gordon M. Cragg, Paul G. Grothaus, and David J. Newman, Impact of Natural Products on Developing New Anti-Cancer Agents, Chem. Rev. 2009, 109, 3012–3043)
- ↑ "Tykerb/Tyverb Phase III gastric cancer study fails to meet primary endpoint" Archived 2015-01-10 at the Wayback Machine
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Lapatinib ditosylate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-28. Retrieved 2021-10-03.
- "Lapatinib Ditosylate". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 2020-10-20. Retrieved 2021-10-03.
- "Lapatinib Ditosylate". National Cancer Institute. Archived from the original on 2021-10-21. Retrieved 2021-10-03.