Apatinib
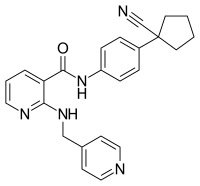 | |
| Clinical data | |
|---|---|
| Other names | Rivoceranib; YN968D1 |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H23N5O |
| Molar mass | 397.482 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Apatinib, also known as Rivoceranib, is a tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor-2 (VEGFR2, also known as KDR). It is an orally bioavailable, small molecule agent which is thought to inhibit angiogenesis in cancer cells; specifically apatinib inhibits VEGF-mediated endothelial cell migration and proliferation thus blocking new blood vessel formation in tumor tissue. This agent also mildly inhibits c-Kit and c-SRC tyrosine kinases.[1]
Apatinib was first synthesized by Advenchen Laboratories in California, USA and licensed out global rights to HLB (Korea) in 2020 and is being developed by Jiangsu Hengrui Medicine (China), LSK BioPartners (US) and HLB Life Science (Korea).[2] It is an investigational cancer drug currently undergoing clinical trials as a potential targeted treatment for metastatic gastric carcinoma, metastatic breast cancer, adenoid cystic carcinoma, and advanced hepatocellular carcinoma.
Phase I/II clinical study
The principal investigator from Fudan University, China, presented results of Phase I/II human clinical studies at the 2009 CSCO Meeting (October 17, 2009). Cancer patients were administered varied doses of Apatinib daily for 28 days. Apatinib was well tolerated at doses below 750 mg/day, 3 of 3 dose limiting toxicities were reported at 1000 mg/day and the maximum tolerated dose is determined to be 850 mg/day. The investigator also reported of 65 cancer patients treated in Phase I/II, 1.54% had a complete response, 12.31% had a partial response, 66.15% had stable disease and 20% had progressive disease.[3]
A separate published report on the safety and pharmacokinetics of apatinib in Human clinical studies concludes that it has encouraging antitumor activity across a broad range of cancer types.[4]
Current status
There is a Phase II/III study recruiting patients in China to determine whether apatinib can improve progression free survival compared with placebo in patients with metastatic gastric carcinoma who have failed two lines of chemotherapy (September, 2009).[5] Apatinib was approved by CFDA in December, 2014 for patients with late-stage gastric carcinoma in China.[6] A phase IV study on safety of Apatinib started in April, 2015. The study aims to recruit 2,000 patients.[7]
As of November, 2010, two additional Phase II clinical studies have been initiated for apatinib in metastatic triple-negative breast cancer patients and advanced hepatocellular carcinoma.[8]
On March 7, 2011, Bukwang announced that it filed an IND to the Korean FDA to begin Human clinical studies of Apatinib in Phase II.[9]
On August, 2018, Bukwang licensed out the commercial rights in Korea for rivoceranib to HLB Life Science [10]
Non-clinical studies
Some cancer cells have the ability to develop resistance to the cytotoxic effects of certain cancer drugs (called multidrug resistance). A study concluded that apatinib may be useful in circumventing cancer cells' multidrug resistance to certain conventional antineoplastic drugs.[11] The study showed that apatinib reverses the ABCB1- and ABCG2-mediated multidrug resistance by inhibiting those functions and increasing the intracellular concentrations of the antineoplastic drugs. This study suggests that apatinib will be potentially effective in combination therapies with conventional anticancer drugs especially in cases where resistance to chemotherapy exists.
References
- ↑ http://www.cancer.gov/Templates/drugdictionary.aspx?CdrID=592508
- ↑ "Apatinib Mesylate (YN968D1)". Archived from the original on 2011-07-13.
- ↑ "Novel Vascular Endothelial Growth Factor Receptor Inhibitor YN968D1 (Apatinib) Against advanced Malignancies: Phase I/II Study". 17 Oct 2009.
- ↑ Li, Jin; Zhao, Xinmin; Chen, Lei; Guo, Haiyi; Lv, Fangfang; Jia, Ka; Yv, Ke; Wang, Fengqing; et al. (2010). "Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies". BMC Cancer. 10: 529. doi:10.1186/1471-2407-10-529. PMC 2984425. PMID 20923544.
- ↑ "A Randomized Phase 2/3 Study of Apatinib as Third Line Treatment in Patients with Metastatic Gastric Carcinoma". 11 July 2011.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "中国晚期胃癌小分子靶向药物研究取得突破". news.sina.com.cn. Retrieved 2015-11-02.
- ↑ "口服小分子靶向药物阿帕替尼开启大样本临床试验 - 丁香园". oncol.dxy.cn. Retrieved 2015-11-02.
- ↑ http://clinicaltrials.gov/ct2/results?term=apatinib
- ↑ "부광약품, 항암제 임상2상 신청". 7 March 2011.
- ↑ "Bukwang Pharm hands over marketing right over gastric cancer drug to HLB - Pulse by Maeil Business News Korea".
- ↑ Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, et al. (2010). "Apatinib (YN968D1) Reverses Multidrug Resistance by Inhibiting the Efflux Function of Multiple ATP-Binding Cassette Transporters". Cancer Research. 70 (20): 7981–7991. doi:10.1158/0008-5472.CAN-10-0111. PMC 2969180. PMID 20876799.