Asciminib
 | |
| Names | |
|---|---|
| Trade names | Scemblix |
| Other names | ABL001 |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor |
| Main uses | Chronic myeloid leukemia which is Philadelphia chromosome-positive (Ph+ CML)[1][2] |
| Side effects | Upper respiratory tract infections, musculoskeletal pain, headache, tiredness, nausea, rash, diarrhea[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
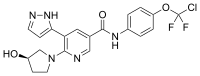
| Formula | C20H18ClF2N5O3 |
| Molar mass | 449.84 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Asciminib, sold under the brand name Scemblix, is a medication used to treat chronic myeloid leukemia which is Philadelphia chromosome-positive (Ph+ CML).[1][2] It is used when other medications have failed.[2] It is taken by mouth twice per day.[2]
Common side effects include upper respiratory tract infections, musculoskeletal pain, headache, tiredness, nausea, rash, and diarrhea.[1] Other side effects may include bone marrow suppression, pancreatitis, high blood pressure, allergic reactions, and heart disease.[1] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor specifically of the BCR::ABL1 tyrosine kinase.[2]
Asciminib was approved for medical use in the United States in 2021 and Europe in 2022.[1][2] In the United Kingdom its costs the NHS for 30 days at 40 mg twice per day £4050 as of 2022.[5] In the United States this amount costs about 18,500 USD.[6]
Medical uses
It is used to treat chronic myeloid leukemia which is Philadelphia chromosome-positive (Ph+ CML).[1][2]
Dosage
It is used at a dose of 40 mg to 200 mg twice daily, depending on the mutations present.[1]
Side effects
Common side effects are symptoms of a cold, muscle pain, joint pain, bone pain, fatigue, nausea, diarrhea, rash as well as the patient displaying abnormal blood tests. [7] Serious side effects of the medication include high blood pressure, low blood cell count, problems with the pancreas, and heart issues. [7] Side effects of the medication on the pancreas may be observed via changes in serum lipase and amylase levels. [8]
Mechanism of action
Asciminib is described as a "STAMP inhibitor," which means "specifically targeting the ABL myristoyl pocket." The wild-type ABL has a myristoylated N-terminus, which binds to an allosteric site, but the ABL fusion protein does not have the myristoylated domain. In the wild-type protein, when myristoylated N-terminus binds to the allosteric site, the kinase has reduced activity. Since the mutant fusion protein does not have the myristoylated N-terminus domain, it is not subject to this form of regulation, and thus the fusion protein is constitutively active. Asciminib binds to the allosteric site, resulting in an inhibition of bcr-abl activity.[9]
Unlike other bcr-abl inhibitors, such as imatinib, asciminib does not bind to the ATP-binding site on the active site of the enzyme. Asciminib and active site bcr-abl inhibitors have non-overlapping resistance mutations. The mutations A337V and P223S overcome the inhibitory activity of asciminib,[10] but asciminib is not affected by the notorious T315I mutation that affects most ATP-competitive active site inhibitors, except ponatinib.
Pharmacodynamics
Asciminib is a substrate of the CYP3A4 enzyme. [8] Asciminib is an inhibitor of CYP3A4, CYP2C9, and P-glycoprotein. [8] Asciminib reaches steady state in 3 days. The volume of distribution of Asciminib is 151 L. [8]
History
The U.S. Food and Drug Administration (FDA) granted the application for asciminib priority review, fast track, orphan drug, and breakthrough therapy designations.[11][12][13]
Society and culture
Legal status
On 23 June 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Scemblix, intended for the treatment of adults with Philadelphia chromosome‑positive chronic myeloid leukemia in chronic phase who have previously been treated with two or more tyrosine kinase inhibitors.[14] The applicant for this medicinal product is Novartis Europharm Limited.[14] Asciminib was approved for medical use in the European Union in August 2022.[2]
References
- 1 2 3 4 5 6 7 8 9 10 "Scemblix- asciminib tablet, film coated". DailyMed. Archived from the original on 5 November 2021. Retrieved 4 November 2021.
- 1 2 3 4 5 6 7 8 9 "Scemblix EPAR". European Medicines Agency (EMA). 20 June 2022. Archived from the original on 9 September 2022. Retrieved 8 September 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 "Scemblix APMDS". Therapeutic Goods Administration (TGA). 26 July 2022. Archived from the original on 2 August 2022. Retrieved 2 August 2022.
- ↑ "Scemblix Product information". Health Canada. Archived from the original on 1 October 2022. Retrieved 30 September 2022.
- ↑ "Asciminib". SPS - Specialist Pharmacy Service. 20 March 2018. Archived from the original on 3 March 2022. Retrieved 25 October 2022.
- ↑ "Scemblix". Archived from the original on 25 October 2022. Retrieved 25 October 2022.
- 1 2 "Asciminib Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 21 June 2022. Retrieved 2022-06-21.
- 1 2 3 4 "Scemblix (asciminib) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Archived from the original on 2 August 2022. Retrieved 2022-06-27.
- ↑ Schoepfer, Joseph; Jahnke, Wolfgang; Berellini, Giuliano; Buonamici, Silvia; Cotesta, Simona; Cowan-Jacob, Sandra W.; Dodd, Stephanie; Drueckes, Peter; Fabbro, Doriano; Gabriel, Tobias; Groell, Jean-Marc; Grotzfeld, Robert M.; Hassan, A. Quamrul; Henry, Chrystèle; Iyer, Varsha; Jones, Darryl; Lombardo, Franco; Loo, Alice; Manley, Paul W.; Pellé, Xavier; Rummel, Gabriele; Salem, Bahaa; Warmuth, Markus; Wylie, Andrew A.; Zoller, Thomas; Marzinzik, Andreas L.; Furet, Pascal (27 September 2018). "Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1". Journal of Medicinal Chemistry. 61 (18): 8120–8135. doi:10.1021/acs.jmedchem.8b01040. PMID 30137981. S2CID 52073282.
- ↑ Jones, Jill K.; Thompson, Eric M. (September 2020). "Allosteric inhibition of ABL kinases: Therapeutic potential in cancer". Molecular Cancer Therapeutics. 19 (9): 1763–1769. doi:10.1158/1535-7163.MCT-20-0069. PMC 7484003. PMID 32606014.
- ↑ "FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia". U.S. Food and Drug Administration (FDA) (Press release). 29 October 2021. Archived from the original on 4 November 2021. Retrieved 4 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Asciminib Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 27 February 2017. Archived from the original on 29 October 2021. Retrieved 29 October 2021.
- ↑ "Novartis receives FDA Breakthrough Therapy designations for investigational STAMP inhibitor asciminib (ABL001) in chronic myeloid leukemia". Novartis (Press release). 8 February 2020. Archived from the original on 29 October 2021. Retrieved 29 October 2021.
- 1 2 "Scemblix: Pending EC decision". European Medicines Agency. 23 June 2022. Archived from the original on 26 June 2022. Retrieved 26 June 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT02081378 for "A Phase I Study of Oral ABL001 in Patients With CML or Ph+ ALL" at ClinicalTrials.gov
- Clinical trial number NCT03106779 for "Study of Efficacy of CML-CP Patients Treated With ABL001 Versus Bosutinib, Previously Treated With 2 or More TKIs" at ClinicalTrials.gov