Lenvatinib
 | |
| Names | |
|---|---|
| Trade names | Lenvima, Kisplyx, others |
| Other names | E7080 |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Thyroid cancer, renal cell cancer, hepatocellular carcinoma[2][3] |
| Side effects | High blood pressure, diarrhea, weight loss, nausea, inflammation of the mouth, headache, rash[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 85% (estimated) |
| Protein binding | 98–99% |
| Metabolism | CYP3A4, aldehyde oxidase, non-enzymatic |
| Metabolites | Desmethyl-lenvatinib (M2) and others |
| Elimination half-life | 28 hours |
| Excretion | ~65% feces, 25% urine |
| Chemical and physical data | |
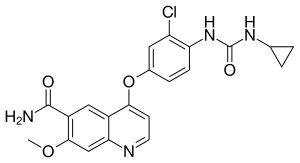
| Formula | C21H19ClN4O4 |
| Molar mass | 426.86 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Lenvatinib, sold under the brand name Lenvima among others, is medication used to treat certain types of thyroid cancer, renal cell cancer, and hepatocellular carcinoma.[2][3] For thyroid cancer, it is used when radioactive iodine is not effective.[1] It is taken by mouth.[1]
Common side effects include high blood pressure, diarrhea, weight loss, nausea, inflammation of the mouth, headache, and rash.[2] Other side effects may include kidney problems, heart failure, blood clots, bleeding in the brain, and liver problems.[2] Use in pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor against VEGFR1, VEGFR2 and VEGFR3.[1]
Lenvatinib was approved for medical use in the United States and Europe in 2015.[1][2] In the United Kingdom it costs the NHS about £1,400 at a dose of 10 mg per day for a month as of 2021.[3] In the United States this amount is about 21,300 USD.[4]
Medical uses
Lenvatinib is approved (since 2015) for the treatment of differentiated thyroid cancer that is either locally recurrent or metastatic, progressive, and did not respond to treatment with radioactive iodine (radioiodine).[5][6]
In May 2016, the U.S. Food and Drug Administration (FDA) approved it (in combination with everolimus) for the treatment of advanced renal cell carcinoma following one prior anti-angiogenic therapy.[7]
The drug is also approved in the US and in the European Union for hepatocellular carcinoma that cannot be removed surgically in patients who have not received cancer therapy by mouth or injection.[8][9]
Dosage
It is used at a dose of 8 mg to 24 mg once per day.[3]
Side effects
Hypertension (high blood pressure) was the most common side effect in studies (73% of patients, versus 16% in the placebo group), followed by diarrhoea (67% vs. 17%) and fatigue (67% vs. 35%).[6] Other common side effects included decreased appetite, hypotension (low blood pressure), thrombocytopenia (low blood platelet count), nausea, muscle and bone pain.[5]
Interactions
As lenvatinib moderately prolongs QT time, addition of other drugs with this property could increase the risk of a type of abnormal heart rhythm, namely torsades de pointes. No relevant interactions with enzyme inhibitors and inducers are expected.[6]
Pharmacology
Mechanism of action
Lenvatinib acts as a multiple kinase inhibitor. It inhibits the three main vascular endothelial growth factor receptors VEGFR1, 2 and 3, as well as fibroblast growth factor receptors (FGFR) 1, 2, 3 and 4, platelet-derived growth factor receptor (PDGFR) alpha, c-Kit, and the RET proto-oncogene. Some of these proteins play roles in cancerogenic signalling pathways. VEGFR2 inhibition is thought to be the main reason for the most common side effect, hypertension.[5]
Pharmacokinetics
Lenvatinib is absorbed quickly from the gut, reaching peak blood plasma concentrations after one to four hours (three to seven hours if taken with food). Bioavailability is estimated to be about 85%. The substance is almost completely (98–99%) bound to plasma proteins, mainly albumin.[5]
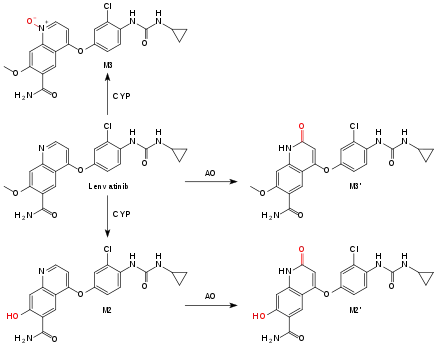
Lenvatinib is metabolized by the liver enzyme CYP3A4 to desmethyl-lenvatinib (M2). M2 and lenvatinib itself are oxidized by aldehyde oxidase (AO) to substances called M2' and M3',[10] the main metabolites in the feces. Another metabolite, also mediated by a CYP enzyme, is the N-oxide M3. Non-enzymatic metabolization also occurs, resulting in a low potential for interactions with enzyme inhibitors and inducers.[5]
Terminal half-life is 28 hours, with about two thirds being excreted via the feces, and one quarter via the urine.[5]
Chemistry
Lenvatinib is used in form of the mesylate salt (CAS number 857890-39-2 ).
History
A phase I clinical trial in cancer patients was performed in 2006.[11] A phase III trial treating thyroid cancer patients started in March 2011.[12]
Lenvatinib was granted orphan drug status for treatment of various types of thyroid cancer that do not respond to radioiodine in the US and Japan in 2012 and in Europe in 2013.[13]
In February 2015, the U.S. FDA approved lenvatinib for treatment of progressive, radioiodine refractory differentiated thyroid cancer.[14] In May 2015, European Medicines Agency (EMA) approved the drug for the same indication.[15]
In May 2016, the FDA approved it (in combination with everolimus) for the treatment of advanced renal cell carcinoma following one prior anti-angiogenic therapy.[7]
In August 2018, the FDA approved lenvatinib for the first-line treatment of people with unresectable hepatocellular carcinoma (HCC).[9]
Brand names
In Bangladesh under the trade name Lenvanix.
References
- 1 2 3 4 5 6 "Lenvatinib Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 21 November 2021.
- 1 2 3 4 5 6 "Lenvima". Archived from the original on 24 June 2021. Retrieved 21 November 2021.
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1033. ISBN 978-0857114105.
- ↑ "Lenvima Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 January 2021. Retrieved 21 November 2021.
- 1 2 3 4 5 6 Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
{{cite book}}: CS1 maint: unrecognized language (link) - 1 2 3 FDA Professional Drug Information for Lenvima.
- 1 2 "Lenvatinib in combination with Everolimus". U.S. Food and Drug Administration (FDA). 16 May 2016. Archived from the original on 23 April 2019. Retrieved 15 May 2021.
- ↑ "Lenvima". European Medicines Agency. 2015-05-28. Archived from the original on 2021-06-24. Retrieved 2021-05-15.
- 1 2 "FDA approves lenvatinib for unresectable hepatocellular carcinoma". U.S. Food and Drug Administration (FDA). 16 August 2018. Archived from the original on 2019-09-28. Retrieved 2018-08-16.
- 1 2 Inoue K, Mizuo H, Kawaguchi S, Fukuda K, Kusano K, Yoshimura T (August 2014). "Oxidative metabolic pathway of lenvatinib mediated by aldehyde oxidase". Drug Metabolism and Disposition. 42 (8): 1326–33. doi:10.1124/dmd.114.058073. PMID 24914245. S2CID 206497491.
- ↑ Glen H, Boss D, Evans TR, Roelvink M, et al. (2007). "A phase I dose finding study of E7080 in patients (pts) with advanced malignancies". Journal of Clinical Oncology, ASCO Annual Meeting Proceedings Part I. 25 (18S): 14073. Archived from the original on 2012-02-24. Retrieved 2021-05-15.
- ↑ Clinical trial number NCT01321554 for "A Trial of E7080 in 131I-Refractory Differentiated Thyroid Cancer" at ClinicalTrials.gov
- ↑ "Phase III trial shows lenvatinib meets primary endpoint of progression free survival benefit in treatment of radioiodine-refractory differentiated thyroid cancer" (PDF). Eisai. 3 February 2014. Archived (PDF) from the original on 5 March 2016. Retrieved 15 May 2021.
- ↑ U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. Archived 2019-04-23 at the Wayback Machine
- ↑ "Summary of the European public assessment report (EPAR) for Lenvima". European Medicines Agency. Archived from the original on 2018-06-20. Retrieved 2022-03-14.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Lenvatinib mesylate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-11-12. Retrieved 2021-05-15.