Pazopanib
 | |
| Names | |
|---|---|
| Pronunciation | /pæˈzoʊpənɪb/ paz-OH-pə-nib |
| Trade names | Votrient |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Renal cell carcinoma, soft tissue sarcoma[2] |
| Side effects | Diarrhea, high blood pressure, white hair, nausea, tiredness, abdominal pain, low blood cells, liver problems[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| Typical dose | 800 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610013 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 21% (14–39%)[3] |
| Protein binding | >99.5%[3][4] |
| Metabolism | Liver: CYP3A4 (major), 1A2 and 2C8 (minor)[4] |
| Elimination half-life | 30.9±4 hours[3] |
| Excretion | Faeces (primary), urine (<4%)[4] |
| Chemical and physical data | |
| Formula | C21H23N7O2S |
| Molar mass | 437.52 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pazopanib, sold under the brand name Votrient, is a medication used to treat renal cell carcinoma (RCC) and certain soft tissue sarcoma.[2] In RCC it is used when other treatments have failed.[2] It is taken by mouth.[2]
Common side effects include diarrhea, high blood pressure, white hair, nausea, tiredness, abdominal pain, low blood cells, and liver problems.[1] Other side effects may include low thyroid, QT prolongation, bleeding, blood clots, and gastrointestinal perforation.[1] Use during pregnancy may harm the baby.[1] It is a tyrosine kinase inhibitor.[1]
Pazopanib was approved for medical use in the United States in 2009.[1] In Europe it received conditional approval in 2010 and full approval in 2013.[5] In the United Kingdom it costs the NHS about £2,250 per month as of 2021.[2] In the United States this amount costs about 14,200 USD.[6]
Medical uses
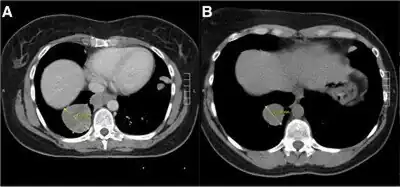
It is used for advanced/metastatic renal cell carcinoma and advanced soft tissue sarcomas.[4][7][8][9][10] In Australia and New Zealand, it is subsidised under the Pharmaceutical Benefits Scheme (PBS) and by Pharmac respectively, under a number of conditions, including:[11][12]
- The medication is used to treat clear cell renal cell carcinoma, the most common histological subtype.
- The treatment phase is continuing treatment beyond 3 months.
- The patient has been issued an authority prescription for pazopanib.
- The patient must have stable or responding disease according to the Response Evaluation Criteria In Solid Tumours (RECIST).
- This treatment must be the sole tyrosine kinase inhibitor subsidised for this condition.
Dosage
The typical dose is 800 mg per day.[2] Lower doses may be used if intolerance.[2]
Contraindications
The only contraindication is hypersensitivity to pazopanib or any of its excipients.[9] Cautions include:[4]
- Hypertension, including hypertensive crises have been reported.
- QT interval prolongation and torsades de pointes have been reported.
- Thrombotic microangiopathy has been reported.
- Thrombotic thrombocytopenic purpura has been reported.
- Haemolytic uremic syndrome has been reported.
- Haematologic parameter alterations have been reported in 31–37% of patients.
- Events of cardiac dysfunction (decreased left ventricular ejection fraction and congestive heart failure) have been observed.
- Fatal haemorrhage, arterial and venous thrombotic events and perforations in the gastrointestinal tract have been observed in randomized clinical trials.
It has one black box warning by the US FDA, namely severe hepatotoxicity including fatalities.[4][7]
Side effects
The most common side effects of pazopanib are nausea, vomiting, diarrhoea (occurs in about half of patients), changes in hair colour, hypertension (which usually occurs during the first few weeks of treatment), appetite loss, hyperglycaemia, hypoglycaemia, electrolyte abnormalities (including hypocalcaemia, hypomagnesemia, hypophosphatemia), laboratory anomalies (including increased AST, ALT and protein in the urine), oedema, hair loss or discolouration, taste changes, abdominal pain, rash, fatigue and bone marrow suppression (including leucopenia, neutropenia, thrombocytopenia and lymphopenia).[13] It has been associated with a low, but real risk of potentially fatal liver damage.[13]
Overdose
The treatment for overdose is purely supportive and the symptoms include grade 3 hypertension and fatigue.[9]
Interactions
Drug interactions include:[4]
- Co-administration with strong inhibitors of the liver enzyme CYP3A4 (e.g. ketoconazole, ritonavir, clarithromycin, grapefruit juice) may increase pazopanib serum levels as it is a CYP3A4 substrate.
- CYP3A4 inducers (e.g. rifampin, carbamazepine) decrease pazopanib serum levels.
- It is a p-glycoprotein (PGP) substrate and hence PGP inhibitors such as quinidine may interact with pazopanib.
- Pazopanib is not a substrate for either of the liver enzymes OATP1B1 and OATP1B3.[14]
- Pazopanib has inhibitory potency towards OATP1B1 but not for OATP1B3.[15]
Pharmacology
Mechanism of action
Pazopanib is a multiple kinase inhibitor that limits tumor growth by targeting angiogenesis via inhibition of enzymes including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), c-KIT and FGFR.[4][13][16][17][18][19]
Pharmacokinetics
After oral intake of a single tablet, pazopanib has a bioavailability of 21% with a range of 14–39% between people. It reaches highest concentrations in the blood plasma after median 3.5 hours; the range in studies was 1.0 to 11.9 hours. When taken regularly, the area under the curve (AUC) increases 1.23- to 4-fold as compared to a single dose. Taking the drug together with food approximately doubles the AUC as well as the highest plasma concentrations (Cmax); and crushing the tablet increases the AUC 1.46-fold, as well doubling the Cmax.[3][8]
When in the bloodstream, more than 99.5% of the substance are bound to plasma proteins. The liver enzyme mainly responsible for metabolizing the drug is CYP3A4; and there are minor contributions from CYP1A2 and CYP2C8. Metabolites identified in tests with human liver cells and microsomes include various hydroxyl derivatives and possibly a carboxylic acid. Only 6% of the circulating substance is in the form of metabolites, and all but one of them are 10- to 20-fold less active than pazopanib itself. Consequently, the metabolites are not considered important for the drug's therapeutic effect.[3][8]
Pazopanib is eliminated with a biological half-life of 30.9±4 hours on average (range 21–51 hours) mainly via the faeces. Less than 4% are eliminated via the urine.[3][8]
History
It is approved by the US Food and Drug Administration (FDA) (19 October 2009), the European Union's European Medicines Agency (EMA) (14 June 2010), the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) (14 June 2010) and Australia's Therapeutic Goods Administration (TGA) (30 June 2010).[4][7][8][9][10]
References
- 1 2 3 4 5 6 7 "PAZOPanib Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 26 October 2021.
- 1 2 3 4 5 6 7 8 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1039. ISBN 978-0857114105.
- 1 2 3 4 5 6 "CHMP Assessment Report: Votrient (pazopanib)" (PDF). European Medicines Agency. Archived (PDF) from the original on 9 October 2016. Retrieved 8 October 2016.
- 1 2 3 4 5 6 7 8 9 "Votrient (pazopanib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 27 January 2014.
- ↑ "Votrient". Archived from the original on 22 October 2021. Retrieved 26 October 2021.
- ↑ "Pazopanib Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 26 October 2021.
- 1 2 3 "Votrient- pazopanib hydrochloride tablet, film coated". DailyMed. 17 August 2020. Archived from the original on 10 November 2020. Retrieved 9 November 2020.
- 1 2 3 4 5 "Votrient : EPAR - Product Information" (PDF). European Medicines Agency. Glaxo Group Ltd. 23 January 2014. Archived (PDF) from the original on 4 February 2014. Retrieved 27 January 2014.
- 1 2 3 4 "Votrient 200 mg and 400 mg film coated tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. GlaxoSmithKline UK. 20 December 2013. Archived from the original on 1 February 2014. Retrieved 27 January 2014.
- 1 2 "Product Information Votrient Tablets" (PDF). TGA eBusiness Services. GlaxoSmithKline Australia Pty Ltd. 25 March 2013. Archived from the original on 25 June 2016. Retrieved 27 January 2014.
- ↑ "Pharmaceutical Benefits Scheme (PBS) - Pazopanib". Pharmaceutical Benefits Scheme. Australian Government. Archived from the original on 1 February 2014. Retrieved 27 January 2014.
- ↑ "Pazopanib - Online Pharmaceutical Schedule". Pharmaceutical Management Agency. Archived from the original on 11 June 2016. Retrieved 9 June 2015.
- 1 2 3 Zivi, A; Cerbone, L; Recine, F; Sternberg, CN (September 2012). "Safety and tolerability of pazopanib in the treatment of renal cell carcinoma". Expert Opinion on Drug Safety. 11 (5): 851–859. doi:10.1517/14740338.2012.712108. PMID 22861374. S2CID 2178331.
- ↑ Khurana V, Minocha M, Pal D, Mitra AK (March 2014). "Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors". Drug Metabol Drug Interact. 29 (3): 179–90. doi:10.1515/dmdi-2013-0062. PMC 4407685. PMID 24643910.
- ↑ Khurana V, Minocha M, Pal D, Mitra AK (May 2014). "Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors". Drug Metabol Drug Interact. 29 (4): 249–59. doi:10.1515/dmdi-2014-0014. PMC 4407688. PMID 24807167.
- ↑ Verweij, J; Sleijfer, S (May 2013). "Pazopanib, a new therapy for metastatic soft tissue sarcoma". Expert Opinion on Pharmacotherapy. 14 (7): 929–935. doi:10.1517/14656566.2013.780030. PMID 23488774. S2CID 5063031.
- ↑ Schöffski, P (June 2012). "Pazopanib in the treatment of soft tissue sarcoma". Expert Review of Anticancer Therapy. 12 (6): 711–723. doi:10.1586/era.12.41. PMID 22716487. S2CID 1738165.
- ↑ Pick, AM; Nystrom, KK (March 2012). "Pazopanib for the treatment of metastatic renal cell carcinoma". Clinical Therapeutics. 34 (3): 511–520. doi:10.1016/j.clinthera.2012.01.014. PMID 22341567.
- ↑ Rimel, BJ (April 2015). "Antiangiogenesis agents in ovarian cancer". Contemporary Oncology. 7 (2): 16–19. Archived from the original on 2018-09-20. Retrieved 2021-08-24.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Pazopanib hydrochloride". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-11-10. Retrieved 2021-08-24.