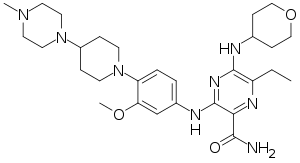
Gilteritinib
 | |
| Names | |
|---|---|
| Trade names | Xospata |
IUPAC name
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Acute myeloid leukemia (AML)[1] |
| Side effects | Liver problems, tiredness, diarrhea, nausea, cough, swelling, shortness of breath, low blood pressure, pain[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | By mouth |
| Typical dose | 120 mg OD[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619003 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C29H44N8O3 |
| Molar mass | 552.724 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Gilteritinib, sold under the brand name Xospata, is a medication used to treat acute myeloid leukemia (AML).[1] Specifically it is used for cases that have failed other treatments and have a specific mutation in FLT3.[4] It is taken by mouth.[4] It may require six months for benefits to occur.[8]
Common side effects include liver problems, tiredness, diarrhea, nausea, cough, swelling, shortness of breath, low blood pressure, and pain.[1] Other side effects may include differentiation syndrome, arrhythmias, anaphylaxis, and posterior reversible encephalopathy syndrome (PRES).[1][4] Use in pregnancy may harm the baby.[4] It is a tyrosine kinase inhibitor that blocks FLT3.[1]
Gilteritinib was approved for medical use in the United States in 2018, Europe in 2019, and Australia in 2020.[6][1][9] In the United Kingdom 4 weeks of treatment costs the NHS about £14,200 as of 2021.[8] In the United States this amount costs about 23,700 USD.[10]
Medical uses
Dosage
It is generally taken at a dose of 120 mg per day.[4] Doses of up to 200 mg per day may be used if no response to smaller doses.[8]
History
In April 2018, Astellas filed a new drug application with the Food and Drug Administration for gilteritinib for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory acute myeloid leukemia (AML).[11]
Gilteritinib was granted orphan drug status in the United States, Europe, and Japan.[12]
Research
Gilteritinib has been repurposed as a potential antiviral drug as a possible application in the treatment of COVID-19.[13]
References
- 1 2 3 4 5 6 7 8 "Xospata". Archived from the original on 9 December 2020. Retrieved 3 December 2021.
- 1 2 "Xospata Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). 11 April 2020. Archived from the original on 10 December 2020. Retrieved 16 August 2020.
- ↑ "Gilteritinib (Xospata) Use During Pregnancy". Drugs.com. 20 August 2019. Archived from the original on 21 October 2020. Retrieved 16 August 2020.
- 1 2 3 4 5 6 "Gilteritinib Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 3 December 2021.
- ↑ "Xospata 40 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 13 November 2019. Archived from the original on 8 December 2020. Retrieved 16 August 2020.
- 1 2 "Xospata- gilteritinib tablet". DailyMed. 31 May 2019. Archived from the original on 9 December 2020. Retrieved 16 August 2020.
- ↑ "Xospata EPAR". European Medicines Agency (EMA). 16 September 2019. Archived from the original on 9 December 2020. Retrieved 16 August 2020.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1027. ISBN 978-0857114105.
- ↑ "AusPAR: Gilteritinib (as fumarate)". Therapeutic Goods Administration (TGA). 11 September 2020. Archived from the original on 10 December 2020. Retrieved 23 September 2020.
- ↑ "Xospata Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 3 December 2021.
- ↑ "FDA Approval Sought for Gilteritinib in FLT3+ AML". onclive.com. April 24, 2018. Archived from the original on September 29, 2018. Retrieved September 29, 2018.
- ↑ "U.S. FDA Grants Priority Review to Astellas' New Drug Application for Gilteritinib for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML)". Drugs.com. Archived from the original on 2020-12-09. Retrieved 2018-12-03.
- ↑ Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, et al. (2020-08-06). "The Global Phosphorylation Landscape of SARS-CoV-2 Infection". Cell. 182 (3): 685–712.e19. doi:10.1016/j.cell.2020.06.034. PMC 7321036. PMID 32645325.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Gilteritinib fumarate". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 2021-01-10. Retrieved 2021-05-03.
- "Gilteritinib fumarate". National Cancer Institute. Archived from the original on 2021-01-12. Retrieved 2021-05-03.