Sotorasib
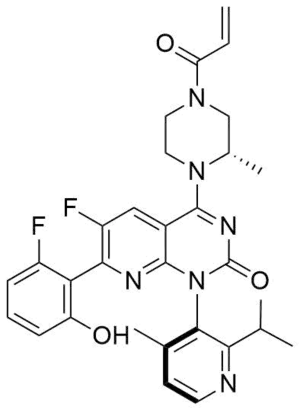 | |
| Names | |
|---|---|
| Trade names | Lumakras, Lumykras |
| Other names | AMG 510 |
IUPAC name
| |
| Clinical data | |
| Main uses | Non-small-cell lung cancer (NSCLC)[1] |
| Side effects | Diarrhea, musculoskeletal pain, nausea, tiredness, liver damage, cough[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 960 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C30H30F2N6O3 |
| Molar mass | 560.606 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sotorasib, sold under the brand names Lumakras and Lumykras, is a medication used to treat non-small-cell lung cancer (NSCLC).[1] Specifically it is used for advanced disease that has a KRAS G12C-mutation and failed other treatments.[1][5] It is taken by mouth.[1]
Common side effects include diarrhea, musculoskeletal pain, nausea, tiredness, liver damage, and cough.[1] Other side effects may include interstitial lung disease.[1] Safety in pregnancy is unclear.[1] It is an inhibitor of the RAS GTPase family, specifically the KRAS p.G12C mutation.[1][6]
Sotorasib was approved for medical use in the United States in 2021 and Europe in 2022.[1][5] In the United Kingdom a month of medication costs the NHS about £6900 as of 2022.[7] This amount in the United States costs about 18,900 USD.[8]
Medical uses
Sotorasib is indicated for the treatment of adults with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy.[1][4]
Dosage
The typical dose is 960 mg once per day.[1] A lower dose may be used in people with certain side effects.[5]
Pharmacology
Sotorasib can exist in either of two atropisomeric forms and one is more active than the other.[9] It selectively forms an irreversible covalent bond to the sulfur atom in the cysteine residue that is present in the mutated form of KRAS, but not in the normal form.[9]
History
It is the first approved targeted therapy for tumors with any KRAS mutation, which accounts for approximately 25% of mutations in non-small cell lung cancers.[4] KRAS G12C mutations occur in about 13% of people with non-small cell lung cancers.[4][10]
Sotorasib is being developed by Amgen. Phase I clinical trials were completed in 2020.[11][12][13] In December 2019, it was approved to begin Phase II clinical trials.[14]
Because the G12C KRAS mutation is relatively common in some cancer types, 14% of non-small-cell lung cancer adenocarcinoma patients and 5% of colorectal cancer patients,[9] and sotorasib is the first drug candidate to target this mutation, there have been high expectations for the drug.[9][15][16] The Food and Drug Administration has granted a fast track designation to sotorasib for the treatment of metastatic non-small-cell lung carcinoma with the G12C KRAS mutation.[17]
Researchers evaluated the efficacy of sotorasib in a study of 124 participants with locally advanced or metastatic KRAS G12C-mutated non-small cell lung cancer with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy.[4] The major outcomes measured were objective response rate (proportion of participants whose tumor is destroyed or reduced) and duration of response.[4] The objective response rate was 36% and 58% of those participants had a duration of response of six months or longer.[4]
The U.S. Food and Drug Administration (FDA) granted the application for sotorasib orphan drug, fast track, priority review, and breakthrough therapy designations.[4] The FDA collaborated with the Australian Therapeutic Goods Administration (TGA), the Brazilian Health Regulatory Agency (ANVISA), Health Canada and the United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA).[4] The application reviews are ongoing at the other regulatory agencies.[4]
The FDA granted approval of Lumakras to Amgen Inc.[4]
Society and culture
Economics
At introduction, in the United States, Sotorasib costs US$17,900 per month.[10]
Legal status
In November 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Lumykras, intended for the treatment of people with KRAS G12C mutation non-small cell lung cancer (NSCLC).[18] The applicant for this medicinal product is Amgen Europe B.V.[18] Sotorasib was approved for medical use in the European Union in January 2022.[5]
Names
Sotorasib is the recommended international nonproprietary name (INN).[19]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Lumakras- sotorasib tablet, coated". DailyMed. Archived from the original on 6 June 2021. Retrieved 6 June 2021.
- 1 2 "Lumakras". Therapeutic Goods Administration (TGA). 13 April 2022. Archived from the original on 12 April 2022. Retrieved 23 April 2022.
- ↑ "Summary Basis of Decision (SBD) for Lumakras". Health Canada. 23 October 2014. Archived from the original on 29 May 2022. Retrieved 29 May 2022.
- 1 2 3 4 5 6 7 8 9 10 11 "FDA Approves First Targeted Therapy for Lung Cancer Mutation Previously Considered Resistant to Drug Therapy". U.S. Food and Drug Administration (FDA). 28 May 2021. Archived from the original on 28 May 2021. Retrieved 28 May 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 "Lumykras EPAR". European Medicines Agency. 10 November 2021. Archived from the original on 23 April 2022. Retrieved 23 April 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "KRAS mutant-targeting AMG 510". NCI Drug Dictionary. National Cancer Institute. 2 February 2011. Archived from the original on 10 July 2019. Retrieved 16 November 2019.
- ↑ "Sotorasib". SPS - Specialist Pharmacy Service. 24 September 2020. Archived from the original on 3 March 2022. Retrieved 29 October 2022.
- ↑ "Sotorasib". Archived from the original on 29 October 2022. Retrieved 29 October 2022.
- 1 2 3 4 Halford B (3 April 2019). "Amgen unveils its KRas inhibitor in human clinical trials: AMG 510 shuts down a mutant version of the cancer target via covalent interaction". Chemical & Engineering News. 97 (4). Archived from the original on 14 April 2019. Retrieved 16 November 2019.
- 1 2 "FDA approves Amgen drug for lung cancer with specific mutation". CNBC. 28 May 2021. Archived from the original on 28 May 2021. Retrieved 28 May 2021.
- ↑ Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. (2020). "KRASG12C inhibition with sotorasib in advanced solid tumors". N Engl J Med. 383 (13): 1207–1217. doi:10.1056/NEJMoa1917239. PMC 7571518. PMID 32955176.
- ↑ Clinical trial number NCT03600883 for "A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of AMG 510 in Subjects With Solid Tumors With a Specific KRAS Mutation " at ClinicalTrials.gov
- ↑ "The Discovery Of Amgen's Novel Investigational KRAS(G12C) Inhibitor AMG 510 Published In Nature" (Press release). Amgen. 30 October 2019. Archived from the original on 16 November 2019. Retrieved 16 November 2019.
- ↑ Irving M (24 December 2019). "Drug targeting common cancer cause enters phase 2 clinical trials". New Atlas. Archived from the original on 24 December 2019. Retrieved 24 December 2019.
- ↑ Al Idrus A (9 September 2019). "Amgen's KRAS drug continues to deliver but faces 'curse' of high expectations". fiercebiotech.com. Archived from the original on 25 September 2022. Retrieved 16 November 2019.
- ↑ Kaiser J (October 2019). "Two new drugs finally hit 'undruggable' cancer target, providing hope for treatments". Science. doi:10.1126/science.aba0472.
- ↑ Astor L (9 September 2019). "FDA Grants AMG 510 Fast Track Designation for KRAS G12C+ NSCLC". targetedonc.com. Archived from the original on 16 November 2019. Retrieved 16 November 2019.
- 1 2 "Lumykras: Pending EC decision". European Medicines Agency. 11 November 2021. Archived from the original on 13 November 2021. Retrieved 13 November 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ World Health Organization (2021). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 85" (PDF). WHO Drug Information. 35 (1). Archived (PDF) from the original on 19 April 2021. Retrieved 28 May 2021.
Further reading
- Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. (September 2020). "KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors". N Engl J Med. 383 (13): 1207–17. doi:10.1056/NEJMoa1917239. PMC 7571518. PMID 32955176.
- Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, et al. (January 2020). "Discovery of a Covalent Inhibitor of KRASG12C (AMG 510) for the Treatment of Solid Tumors". J Med Chem. 63 (1): 52–65. doi:10.1021/acs.jmedchem.9b01180. PMID 31820981.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT03600883 for "A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of AMG 510 in Subjects With Solid Tumors With a Specific KRAS Mutation (CodeBreaK 100)" at ClinicalTrials.gov