Pexidartinib
 | |
| Names | |
|---|---|
| Trade names | Turalio |
| Other names | PLX-3397 |
IUPAC name
| |
| Clinical data | |
| Drug class | Kinase inhibitor[1] |
| Main uses | Tenosynovial giant cell tumor (TGCT)[1] |
| Side effects | Liver problems, loss of hair color, tiredness, low neutrophils, increased cholesterol, eye swelling, rash[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619050 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
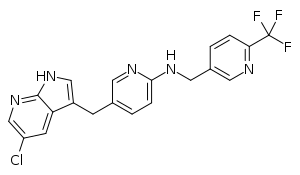
| Formula | C20H15ClF3N5 |
| Molar mass | 417.82 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pexidartinib, sold under the brand name Turalio, is a medication used to treat tenosynovial giant cell tumor (TGCT).[1] It is used in cases which result in significant problems and cannot be treated by surgery.[1] It is taken by mouth.[1]
Common side effects include liver problems, loss of hair color, tiredness, low neutrophils, increased cholesterol, eye swelling, and rash.[2] The liver problems can result in death.[2] Use in pregnancy may harm the baby.[1] It is a kinase inhibitor and works by blocking colony-stimulating factor-1 receptor (CSF-1R).[1]
Pexidartinib was approved for medical use in the United States in 2019.[1] It was refused approval in Europe in 2020 due to minimal benefits and concerns regarding side effects.[3] It is not approved in the United Kingdom.[4] In the United States it costs about 21,200 USD per month.[5]
Medical uses
Dosage
It is taken at a dose of 400 mg twice per day.[1]
History
The approval of pexidartinib was based on the results of a trial of 120 subjects, 59 of whom received placebo.[6] The primary efficacy endpoint was the overall response rate (ORR) analyzed after 25 weeks of treatment.[6] The clinical trial demonstrated a statistically significant improvement in ORR in subjects who received pexidartinib, with an ORR of 38%, compared to no responses in subjects who received placebo.[6] The complete response rate was 15% and the partial response rate was 23%.[6] A total of 22 out of 23 responders who had been followed for a minimum of six months following the initial response maintained their response for six or more months, and a total of 13 out of 13 responders who had been followed for a minimum of 12 months following the initial response maintained their response for 12 or more months.[6]
The U.S. Food and Drug Administration (FDA) granted the application for pexidartinib breakthrough therapy designation, orphan drug designation, and priority review designation.[6] The FDA granted the approval of Turalio to Daiichi Sankyo.[6]
Pexidartinib is available in the US only through the Turalio Risk Evaluation and Mitigation Strategy (REMS) Program.[6] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[7]
References
- 1 2 3 4 5 6 7 8 9 "Pexidartinib Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 27 October 2021.
- 1 2 3 "DailyMed - TURALIO- pexidartinib capsule". dailymed.nlm.nih.gov. Archived from the original on 24 October 2020. Retrieved 27 October 2021.
- ↑ "Turalio". Archived from the original on 27 October 2021. Retrieved 27 October 2021.
- ↑ "Pexidartinib". SPS - Specialist Pharmacy Service. 12 January 2016. Archived from the original on 27 October 2021. Retrieved 27 October 2021.
- ↑ "Turalio Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 January 2021. Retrieved 27 October 2021.
- 1 2 3 4 5 6 7 8 "FDA approves first therapy for rare joint tumor". FDA (Press release). 2 August 2019. Archived from the original on 14 September 2019. Retrieved 17 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Archived from the original on 16 September 2020. Retrieved 15 September 2020.
External links
- "Pexidartinib". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. October 2019. PMID 31869194. NBK551730. Archived from the original on 2016-11-23. Retrieved 2021-07-27.
- Lamb YN (November 2019). "Pexidartinib: First Approval". Drugs. 79 (16): 1805–1812. doi:10.1007/s40265-019-01210-0. PMC 7044138. PMID 31602563.
- Roskoski R (February 2020). "Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update". Pharmacol. Res. 152: 104609. doi:10.1016/j.phrs.2019.104609. PMID 31862477.
| External sites: |
|
|---|---|
| Identifiers: |