Isobutyric acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylpropanoic acid[2] | |||
| Other names
Isobutyric acid 2-Methylpropionic acid Isobutanoic acid | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.087 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2529 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C4H8O2 | ||
| Molar mass | 88.11 g/mol | ||
| Density | 0.9697 g/cm3 (0 °C) | ||
| Melting point | −47 °C (−53 °F; 226 K) | ||
| Boiling point | 155 °C (311 °F; 428 K) | ||
| Acidity (pKa) | 4.86[3] | ||
Magnetic susceptibility (χ) |
-56.06x10−6 cm3/mol | ||
| Hazards[4][5] | |||
| GHS labelling: | |||
Pictograms |
 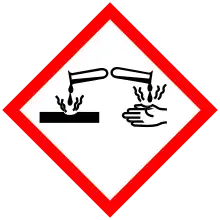  | ||
Signal word |
Danger | ||
Hazard statements |
H226, H302, H311, H314
P210 - P280 - P301 + P312 + P330 - P303 + P361 + P353 - P305 + P351 + P338 + P310 | ||
Precautionary statements |
P210, P280, P301+P312+P330, P303+P361+P353, P305+P351+P338+P310 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Isobutyric acid, also known as 2-methylpropanoic acid or isobutanoic acid, is a carboxylic acid with structural formula (CH3)2CHCOOH. It is an isomer of n-butyric acid. It is classified as a short-chain fatty acid. Deprotonation or esterification gives derivatives called isobutyrates.
Isobutyric acid is a colorless liquid with a somewhat unpleasant odor. It is soluble in water and organic solvents. It is found naturally in carobs (Ceratonia siliqua), in vanilla, and in the root of Arnica dulcis, and as an ethyl ester in croton oil.[6]
Production
Isobutyric acid is manufactured by the oxidation of isobutyraldehyde, which is a byproduct of the hydroformylation of propylene.[7]
It can also be prepared by the high pressure hydrocarboxylation (Koch reaction) from propylene:[7]
- CH3CH=CH2 + CO + H2O → (CH3)2CHCO2H
Isobutyric acid can also be manufactured commercially using engineered bacteria with a sugar feedstock.[8]
Laboratory methods
Many routes are known including the hydrolysis of isobutyronitrile with alkalis and the oxidation of isobutanol with potassium dichromate in the presence of sulfuric acid.[9] In the presence of proton donors, the action of sodium amalgam on methacrylic acid also gives isobutyric acid.[6]
Reactions
The acid reacts as a typical carboxylic acid: it can form amide, ester, anhydride, and chloride derivatives.[10] Its acid chloride is commonly used as the intermediate to obtain the others. When heated with a chromic acid solution it is oxidized to acetone. Alkaline potassium permanganate oxidizes it to α-hydroxyisobutyric acid, (CH3)2C(OH)-CO2H.[6]
Uses
Isobutyric acid and its volatile esters are present naturally in a wide variety of foods and, at varying concentrations, can impart a range of flavors.[11] The compound's safety as a food additive was reviewed by an FAO and WHO panel, who concluded that there were no concerns at the likely levels of intake.[12]
Biology
In humans, isobutyric acid is a minor product of the gut microbiome and can also be produced by metabolism of its esters found in food.[13] It has a characteristic odor like rancid butter[14] (4-carbon organic compounds take the root, butyl, which is in turn from butyric which is in turn from the Latin word for butter and the Greek, βούτυρον) but anosmia for it has been reported in about 2.5% of people.[15]
The metabolism of isobutyric acid in plants has been studied.[16]
Isobutyric acid, along with several other short-chain fatty acids collectively known as "copulins," is found abundantly in human vaginal secretions. Levels of isobutyric acid fluctuate throughout the menstrual cycle, and it is hypothesized to act as an indicator of ovulatory status.[17] Similar cycles are observed in chimpanzees.[18]
See also
- Sucrose acetate isobutyrate
References
- ↑ Merck Index, 11th Edition, 5039
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 748. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Bjerrum, J.; et al. (1958). Stability Constants. London: Chemical Society.
- ↑ Sigma-Aldrich. "Isobutyric acid". Retrieved 2020-10-03.
- ↑ "NFPA Hazard Classification". Retrieved 2020-10-03.
- 1 2 3 Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 4 (11th ed.). Cambridge University Press. p. 892.
- 1 2 Riemenschneider, Wilhelm; Bolt, Hermann (2000). Esters, Organic. Ullmann's Encyclopedia of Industrial Chemistry. p. 10. doi:10.1002/14356007.a09_565. ISBN 978-3527306732.
- ↑ "Biological pathways to produce methacrylate". Archived from the original on 2012-05-02. Retrieved 2011-10-07.
- ↑ I. Pierre and E. Puchot (1873). "New Studies on Valerianic Acid and its Preparation on a Large Scale". Ann. Chim. Phys. 28: 366.
- ↑ Jenkins, P. R. (1985). "Carboxylic acids and derivatives". General and Synthetic Methods. Vol. 7. pp. 96–160. doi:10.1039/9781847556196-00096. ISBN 978-0-85186-884-4.
- ↑ "Isobutyric acid". The Good Scents Company. Retrieved 2020-10-03.
- ↑ FAO/WHO Expert Committee on food additives (1998). "Safety evaluation of certain food additives and contaminants". Retrieved 2020-09-30.
- ↑ "Metabocard for isobutyric acid". Human Metabolome Database. 2020-03-26. Retrieved 2020-09-30.
- ↑ FAO (1998). "Specifications for flavourings: isobutyric acid". Retrieved 2020-10-03.
- ↑ Amoore, J. E. (1967). "Specific Anosmia: A Clue to the Olfactory Code". Nature. 214 (5093): 1095–1098. Bibcode:1967Natur.214.1095A. doi:10.1038/2141095a0. PMID 4861233. S2CID 4222453.
- ↑ Lucas, Kerry A.; Filley, Jessica R.; Erb, Jeremy M.; Graybill, Eric R.; Hawes, John W. (2007). "Peroxisomal Metabolism of Propionic Acid and Isobutyric Acid in Plants". Journal of Biological Chemistry. 282 (34): 24980–24989. doi:10.1074/jbc.m701028200. PMID 17580301. S2CID 7143228.
- ↑ Williams, Megan N.; Jacobson, Amy (April 22, 2016). "Effect of Copulins on Rating of Female Attractiveness, Mate-Guarding, and Self-Perceived Sexual Desirability". Evolutionary Psychology. SAGE Publications. 14 (2): 147470491664332. doi:10.1177/1474704916643328. ISSN 1474-7049.
- ↑ Matsumoto-Oda, Akiko; Oda, Ryo; Hayashi, Yukako; Murakami, Hiroshi; Maeda, Norihiko; Kumazaki, Kiyonori; Shimizu, Keiko; Matsuzawa, Tetsuro (2003). "Vaginal Fatty Acids Produced by Chimpanzees during Menstrual Cycles". Folia Primatologica. S. Karger AG. 74 (2): 75–79. doi:10.1159/000070000. ISSN 0015-5713.