Japanese encephalitis
| Japanese encephalitis | |
|---|---|
| Other names: Japanese B encephalitis | |
 | |
| The geographic distribution of Japanese encephalitis (dark green) | |
| Specialty | Infectious disease |
| Symptoms | Headache, fever, vomiting, confusion, seizures[1] |
| Usual onset | 5 to 15 days after infection[1] |
| Causes | Japanese encephalitis virus (spread by mosquitoes) |
| Diagnostic method | Blood or cerebrospinal fluid testing[2] |
| Prevention | Japanese encephalitis vaccine, avoiding mosquito bites[2] |
| Treatment | Supportive care[1] |
| Prognosis | Permanent neurological problems occur in up to half of survivors[2] |
| Frequency | 68,000[2] |
| Deaths | 17,000[2] |
Japanese encephalitis (JE) is an infection of the brain caused by the Japanese encephalitis virus (JEV).[3] While most infections result in little or no symptoms, occasional inflammation of the brain occurs.[3] In these cases, symptoms may include headache, vomiting, fever, confusion and seizures.[1] This occurs about 5 to 15 days after infection.[1]
JEV is generally spread by mosquitoes, specifically those of the Culex type.[2] Pigs and wild birds serve as a reservoir for the virus.[2] The disease mostly occurs outside of cities.[2] Diagnosis is based on blood or cerebrospinal fluid testing.[2]
Prevention is generally with the Japanese encephalitis vaccine, which is both safe and effective.[2] Other measures include avoiding mosquito bites.[2] Once infected, there is no specific treatment, with care being supportive.[1] This is generally carried out in hospital.[1] Permanent problems occur in up to half of people who recover from JE.[2]
The disease occurs in Southeast Asia and the Western Pacific.[2] About 3 billion people live in areas where the disease occurs.[2] About 68,000 symptomatic cases occur a year, with about 17,000 deaths.[2] Often, cases occur in outbreaks.[2] The disease was first described in Japan in 1871.[2] Despite the name, it is rare in Japan due to immunization efforts.[4]
Signs and symptoms
The Japanese encephalitis virus (JEV) has an incubation period of 2 to 26 days.[5] The vast majority of infections are asymptomatic: only 1 in 250 infections develop into encephalitis.[6][7]
Severe rigors may mark the onset of this disease in humans. Fever, headache and malaise are other non-specific symptoms of this disease which may last for a period of between 1 and 6 days.[8]
Signs which develop during the severe encephalitic stage include neck rigidity, cachexia, hemiparesis, convulsions and a raised body temperature between 38–41 °C (100.4–105.8 °F)[9]. Lifelong neurological defects such as deafness, emotional lability and hemiparesis may occur in those who have had central nervous system involvement.[8]
Cause
It is a disease caused by the mosquito-borne Japanese encephalitis virus (JEV).[10]
Virology
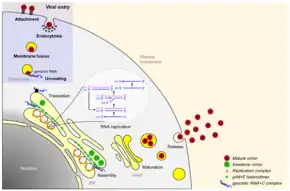
JEV is a virus from the family Flaviviridae, part of the Japanese encephalitis serocomplex of 9 genetically and antigenically related viruses, some which are particularly severe in horses, and four known to infect humans including West Nile virus.[11] The enveloped virus is closely related to the West Nile virus and the St. Louis encephalitis virus. The positive sense single-stranded RNA genome is packaged in the capsid which is formed by the capsid protein. The outer envelope is formed by envelope protein and is the protective antigen. It aids in entry of the virus into the inside of the cell. The genome also encodes several nonstructural proteins (NS1, NS2a, NS2b, NS3, N4a, NS4b, NS5). NS1 is produced as secretory form also. NS3 is a putative helicase, and NS5 is the viral polymerase. It has been noted that Japanese encephalitis infects the lumen of the endoplasmic reticulum (ER)[12][13] and rapidly accumulates substantial amounts of viral proteins.
Based on the envelope gene, there are five genotypes (I–V). The Muar strain, isolated from a patient in Malaya in 1952, is the prototype strain of genotype V. Genotype IV appears to be the ancestral strain, and the virus appears to have evolved in the Indonesian–Malaysian region. [13][14]
Mechanism
Increased microglial activation following Japanese Encephalitis infection has been found to influence the outcome of viral pathogenesis. Microglia are the resident immune cells of the central nervous system (CNS) and have a critical role in host defense against invading microorganisms. Activated microglia secrete cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), which can cause toxic effects in the brain. Additionally, other soluble factors such as neurotoxins, excitatory neurotransmitters, prostaglandin, reactive oxygen, and nitrogen species are secreted by activated microglia.[15][16][17]
Although the net effect of the proinflammatory mediators is to kill infectious organisms and infected cells as well as to stimulate the production of molecules that amplify the mounting response to damage, it is also evident that in a nonregenerating organ such as the brain, a dysregulated innate immune response would be deleterious. In JE the tight regulation of microglial activation appears to be disturbed, resulting in an autotoxic loop of microglial activation that possibly leads to bystander neuronal damage.[18]
Diagnosis
Japanese encephalitis is diagnosed by commercially available tests detecting JE virus-specific IgM antibodies in serum and /or cerebrospinal fluid, for example by IgM capture ELISA.[19]JE virus IgM antibodies are usually detectable 3 to 8 days after onset of illness and persist for 30 to 90 days, but longer persistence has been documented. Therefore, positive IgM antibodies occasionally may reflect a past infection or vaccination. Serum collected within 10 days of illness onset may not have detectable IgM, and the test should be repeated on a convalescent sample. For patients with JE virus IgM antibodies, confirmatory neutralizing antibody testing should be performed.Confirmatory testing in the US is only available at CDC and a few specialized reference laboratories. Viral antigen can be shown in tissues by indirect fluorescent antibody staining.[20][21]
Prevention

Infection with Japanese encephalitis confers lifelong immunity. There are currently three vaccines available: SA14-14-2, IXIARO (IC51, also marketed in Australia, New Zealand as JESPECT and India as JEEV[22]) and ChimeriVax-JE (marketed as IMOJEV).[23] All current vaccines are based on the genotype III virus.[24]
A formalin-inactivated mouse-brain derived vaccine was first produced in Japan in the 1930s and was validated for use in Taiwan in the 1960s and in Thailand in the 1980s. The widespread use of vaccine and urbanization has led to control of the disease in Japan, Korea, Taiwan, and Singapore. The high cost of this vaccine, which is grown in live mice, means that poorer countries have not been able to afford to give it as part of a routine immunization program.[10]The most common adverse effects are redness and pain at the injection site. Uncommonly, an urticarial reaction can develop about four days after injection. Vaccines produced from mouse brain have a risk of autoimmune neurological complications of around 1 per million vaccinations.[25]
The neutralizing antibody persists in the circulation for at least three years, and perhaps longer.[26] Per the Centers for Disease Control and Prevention after receiving the 2 dose vaccine, a booster is recommended for individuals at risk after one year.[27]
Treatment
There is no specific treatment for Japanese encephalitis and treatment is supportive,[28] with assistance given for feeding, breathing or seizure control as required. Raised intracranial pressure may be managed with mannitol.[29]
The effectiveness of intravenous immunoglobulin for the management of encephalitis is unclear due to a lack of evidence.[30] Intravenous immunoglobulin for Japanese encephalitis appeared to have no benefit.[30]
Epidemiology

Japanese encephalitis (JE) is the leading cause of viral encephalitis in Asia, with up to 70,000 cases reported annually.[31] Case-fatality ratio range from 20% to 30% for this virus[32]. Rare outbreaks in U.S. territories in the Western Pacific have also occurred. Residents of rural areas in endemic locations are at highest risk; Japanese encephalitis does not usually occur in urban areas.Countries which have had major epidemics in the past, but which have controlled the disease primarily by vaccination, include China, South Korea, Japan, Taiwan and Thailand. Other countries that still have periodic epidemics include Vietnam, Cambodia, Myanmar, India, Nepal, and Malaysia; Japanese encephalitis has been reported in the Torres Strait Islands. There were reported cases in Kachin State, Myanmar in 2013. The spread of the virus in Australia is of particular concern to Australian health officials due to the unplanned introduction of Culex gelidus, a potential vector of the virus, from Asia. However, the current presence on mainland Australia is minimal. There had been 116 deaths reported in Odisha's backward Malkangiri district of India in 2016.[33][34][35]
Human, cattle, and horses are dead-end hosts as the disease manifests as fatal encephalitis, however pigs act as an amplifying host and have a very important role in the epidemiology of the disease. Infection in swine is asymptomatic, except in pregnant sows, when abortion and fetal abnormalities are common sequelae. The most important vector is Culex tritaeniorhynchus, which feeds on cattle in preference to humans. The natural hosts of the Japanese encephalitis virus are birds, not humans, and many believe the virus will therefore never be completely eliminated.[36] In November 2011, the Japanese encephalitis virus was reported in Culex bitaeniorhynchus in South Korea.[37]
History
The virus appears to have originated from its ancestral virus in the mid-1500s in the Indonesia-Malaysia region and evolved there into five different genotypes and spread across Asia.[38] The mean evolutionary rate has been estimated to be 4.35×10−4 (range: 3.4906×10−4 to 5.303×10−4) nucleotide substitutions per site per year.[38]
Research
A breakthrough in the field of Japanese encephalitis therapeutics is the identification of macrophage receptor involvement in the disease severity. A recent report of an Indian group demonstrates the involvement of monocyte and macrophage receptor CLEC5A in severe inflammatory response in Japanese Encephalitis infection of the brain. This transcriptomic study provides a hypothesis of neuroinflammation and a new lead in development of appropriate therapeutic against Japanese encephalitis.[39]
Recently whole genome microarray research of neurons infected with the Japanese Encephalitis virus has shown that neurons play an important role in their own defense against Japanese Encephalitis infection. Although this challenges the long-held belief that neurons are immunologically quiescent, an improved understanding of the proinflammatory effects responsible for immune-mediated control of viral infection and neuronal injury during Japanese Encephalitis infection is an essential step for developing strategies for limiting the severity of CNS disease.[40]
A number of drugs have been investigated to either reduce viral replication or provide neuroprotection in cell lines or studies upon mice, none are currently advocated in treating human patients:
- The use of rosmarinic acid,[41] arctigenin,[42] and oligosaccharides with degree of polymerization 6 from Gracilaria sp. or Monostroma nitidum[43] have been shown to be effective in a mouse model of Japanese encephalitis.
- Curcumin has been shown to impart neuroprotection against Japanese Encephalitis infection in an in vitro study. Curcumin possibly acts by decreasing cellular reactive oxygen species level, restoration of cellular membrane integrity, decreasing pro-apoptotic signaling molecules, and modulating cellular levels of stress-related proteins. It has also been shown that the production of infective viral particles from previously infected neuroblastoma cells are reduced, which is achieved by the inhibition of ubiquitin-proteasome system.[44]
- Minocycline in mice resulted in marked decreases in the levels of several markers, viral titer, and the level of proinflammatory mediators[45] and also prevents blood brain barrier damage.[46]
References
- 1 2 3 4 5 6 7 "Symptoms and Treatment". CDC. August 2015. Archived from the original on 17 June 2017. Retrieved 29 October 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 "Japanese encephalitis". World Health Organization. December 2015. Archived from the original on 13 July 2017. Retrieved 29 October 2017.
- 1 2 "Japanese Encephalitis". CDC. August 2015. Archived from the original on 24 May 2017. Retrieved 29 October 2017.
- ↑ "Japanese encephalitis - Causes". nhs.uk. 6 February 2019. Archived from the original on 16 August 2020. Retrieved 8 August 2020.
- ↑ Moloney, Rachael M.; Kmush, Brittany; Rudolph, Kara E.; Cummings, Derek A. T.; Lessler, Justin (7 May 2014). "Incubation Periods of Mosquito-Borne Viral Infections: A Systematic Review". The American Journal of Tropical Medicine and Hygiene. 90 (5): 882–891. doi:10.4269/ajtmh.13-0403. PMC 4015582. PMID 24639305.
- ↑ Simon, LV; Kruse, B (January 2018). Encephalitis, Japanese. StatPearls. PMID 29262148. Archived from the original on 28 August 2021. Retrieved 29 July 2018.
- ↑ Peterson, Phillip K.; Toborek, Michal (8 July 2014). Neuroinflammation and Neurodegeneration. Springer. p. 317. ISBN 978-1-4939-1071-7. Archived from the original on 28 August 2021. Retrieved 10 June 2021.
- 1 2 Kumar, Parveen; Clark, Michael L. (29 June 2016). Kumar and Clark's Clinical Medicine E-Book. Elsevier Health Sciences. p. 262. ISBN 978-0-7020-6600-9. Archived from the original on 28 August 2021. Retrieved 1 June 2021.
- ↑ Navalgund, R. R.; Kumar, A. Senthil; Nandy, Subrata (18 October 2018). Remote Sensing of Northwest Himalayan Ecosystems. Springer. p. 464. ISBN 978-981-13-2128-3. Archived from the original on 28 August 2021. Retrieved 2 June 2021.
- 1 2 Solomon, T. (2006). "Control of Japanese encephalitis – within our grasp?". New England Journal of Medicine. 355 (9): 869–71. doi:10.1056/NEJMp058263. PMID 16943399.
- ↑ Lobigs M, Diamond MS (2012). "Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex". Expert Rev Vaccines. 11 (2): 177–87. doi:10.1586/erv.11.180. PMC 3337329. PMID 22309667.
- ↑ He B (March 2006). "Viruses, endoplasmic reticulum stress, and interferon responses". Cell Death Differ. 13 (3): 393–403. doi:10.1038/sj.cdd.4401833. PMID 16397582.
- 1 2 Mahy, B.W.J; Regenmortal, Marc (2008). Encyclopedia of virology (3rd ed.). Amsterdam: Academic Press. pp. 182–188. ISBN 9780123744104. Archived from the original on 8 June 2021. Retrieved 4 June 2021.
- ↑ Clements, Alan N. (1 January 2011). The Biology of Mosquitoes: Viral, Arboviral and Bacterial Pathogens. CABI. p. 252. ISBN 978-1-84593-243-5. Archived from the original on 28 August 2021. Retrieved 10 June 2021.
- ↑ Dutta, Kallol; Rangarajan, Pundi N.; Vrati, Sudhanshu; Basu, Anirban (2010). "Japanese encephalitis: pathogenesis, prophylactics and therapeutics". Current Science. 98 (3): 326–334. ISSN 0011-3891. Retrieved 5 June 2021.
- ↑ Hsieh, Justin T.; St. John, Ashley L. (2 April 2020). "Japanese encephalitis virus and its mechanisms of neuroinvasion". PLoS Pathogens. 16 (4). doi:10.1371/journal.ppat.1008260. ISSN 1553-7366. Archived from the original on 28 August 2021. Retrieved 8 June 2021.
- ↑ Tandon, Prakash Narain; Ramamurthi, Ravi (2012). Textbook of Neurosurgery. JP Medical Ltd. p. 835. ISBN 978-93-5025-072-3. Archived from the original on 28 August 2021. Retrieved 10 June 2021.
- ↑ Thongtan, Thananya; Thepparit, Chutima; Smith, Duncan R. (2012). "The Involvement of Microglial Cells in Japanese Encephalitis Infections". Clinical and Developmental Immunology. 2012. doi:10.1155/2012/890586. ISSN 1740-2522. Archived from the original on 28 August 2021. Retrieved 6 June 2021.
- ↑ Shrivastva A, Tripathi NK, Parida M, Dash PK, Jana AM, Lakshmana Rao PV (2008). "Comparison of a dipstick enzyme-linked immunosorbent assay with commercial assays for detection of Japanese encephalitis virus-specific IgM antibodies". J Postgrad Med. 54 (3): 181–5. doi:10.4103/0022-3859.40959. PMID 18626163.
- ↑ "Diagnostic Testing | Japanese Encephalitis | CDC". www.cdc.gov. 8 February 2019. Archived from the original on 23 March 2021. Retrieved 6 June 2021.
- ↑ "Manual for the Laboratory Diagnosis of Japanese Encephalitis Virus Infection" (PDF). World Health Organization. WHO.int. Archived (PDF) from the original on 15 July 2020. Retrieved 8 June 2021.
- ↑ "Jeev an inactivated Japanese Encephalitis vaccine launched in Hyderabad". pharmabiz.com. 15 September 2012. Archived from the original on 23 October 2012. Retrieved 11 January 2013.
- ↑ Schiøler KL, Samuel M, Wai KL (2007). "Vaccines for preventing Japanese encephalitis". Cochrane Database Syst Rev (3): CD004263. doi:10.1002/14651858.CD004263.pub2. PMC 6532601. PMID 17636750.
- ↑ Hegde, Nagendra R.; Gore, Milind M. (22 February 2017). "Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease". Human Vaccines & Immunotherapeutics. 13 (6): 1320–1337. doi:10.1080/21645515.2017.1285472. ISSN 2164-5515. Archived from the original on 8 May 2020. Retrieved 5 June 2021.
- ↑ Jelinek T (July 2008). "Japanese encephalitis vaccine in travelers". Expert Rev Vaccines. 7 (5): 689–93. doi:10.1586/14760584.7.5.689. PMID 18564023. S2CID 34671998.
- ↑ Kurane I, Takashi T (2000). "Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains". Vaccine. 18 (Suppl): 33–5. doi:10.1016/S0264-410X(00)00041-4. PMID 10821971.
- ↑ "Japanese Encephalitis Vaccine | Japanese Encephalitis | CDC". www.cdc.gov. 25 July 2019. Archived from the original on 6 April 2021. Retrieved 7 June 2021.
- ↑ Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT (2000). "Japanese encephalitis". Journal of Neurology, Neurosurgery, and Psychiatry. 68 (9): 405–15. doi:10.1136/jnnp.68.4.405. PMC 1736874. PMID 10727474.
- ↑ Japanese encephalitis~treatment at eMedicine
- 1 2 Iro, Mildred A.; Martin, Natalie G.; Absoud, Michael; Pollard, Andrew J. (2 October 2017). "Intravenous immunoglobulin for the treatment of childhood encephalitis". The Cochrane Database of Systematic Reviews. 10: CD011367. doi:10.1002/14651858.CD011367.pub2. ISSN 1469-493X. PMC 6485509. PMID 28967695.
- ↑ Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS (November 2011). "Estimmated global incidence of Japanese encephalitis: a systematic review". Bull World Health Organ. 89 (10): 766–74. doi:10.2471/BLT.10.085233. PMC 3209971. PMID 22084515.
- ↑ "Japanese Encephalitis - Chapter 4 - 2020 Yellow Book | Travelers' Health | CDC". wwwnc.cdc.gov. Archived from the original on 10 May 2021. Retrieved 10 June 2021.
- ↑ "WHO | Estimated global incidence of Japanese encephalitis: a systematic review" (PDF). WHO. Archived (PDF) from the original on 9 March 2021. Retrieved 7 June 2021.
- ↑ "WHO World Health Organization: Immunization, Vaccines And Biologicals. Vaccine preventable diseases Vaccines monitoring system 2020 Global Summary Reference Time Series: JAPANESE ENCEPHALITIS". apps.who.int. Archived from the original on 11 November 2020. Retrieved 10 June 2021.
- ↑ Sudeep, A. B. (December 2014). "Culex gelidus: an emerging mosquito vector with potential to transmit multiple virus infections". Journal of Vector Borne Diseases. 51 (4): 251–258. ISSN 0972-9062. Archived from the original on 28 August 2021. Retrieved 10 June 2021.
- ↑ Ghosh D, Basu A (September 2009). Brooker S (ed.). "Japanese encephalitis-a pathological and clinical perspective". PLOS Negl Trop Dis. 3 (9): e437. doi:10.1371/journal.pntd.0000437. PMC 2745699. PMID 19787040.
- ↑ Kim, Heung Chul; Klein, Terry A.; Takhampunya, Ratree; Evans, Brian P.; Mingmongkolchai, Sirima; Kengluecha, Ampornpan; Grieco, John; Masuoka, Penny; Kim, Myung-Soon; Chong, Sung-Tae; Lee, Jong-Koo; Lee, Won-Ja (November 2011). "Japanese encephalitis virus in culicine mosquitoes (Diptera: Culicidae) collected at Daeseongdong, a village in the demilitarized zone of the Republic of Korea". Journal of Medical Entomology. 48 (6): 1250–1256. doi:10.1603/me11091. ISSN 0022-2585. Archived from the original on 28 August 2021. Retrieved 3 June 2021.
- 1 2 Mohammed MA, Galbraith SE, Radford AD, Dove W, Takasaki T, Kurane I, Solomon T (July 2011). "Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype". Infect Genet Evol. 11 (5): 855–62. doi:10.1016/j.meegid.2011.01.020. PMID 21352956.
- ↑ Gupta, Nimesh; Lomash, Vinay; Rao, P. V. Lakshmana (September 2010). "Expression profile of Japanese encephalitis virus induced neuroinflammation and its implication in disease severity". Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology. 49 (1): 4–10. doi:10.1016/j.jcv.2010.06.009. ISSN 1873-5967. Archived from the original on 28 August 2021. Retrieved 7 June 2021.
- ↑ Nimesh Gupta; S.R. Santhosh; J. Pradeep Babu; M.M. Parida; P.V. Lakshmana Rao (January 2010). "Chemokine profiling of Japanese encephalitis virus-infected mouse neuroblastoma cells by microarray and real-time RT-PCR: Implication in neuropathogenesis". Virus Research. 147 (1): 107–12. doi:10.1016/j.virusres.2009.10.018. PMC 7126115. PMID 19896511.
- ↑ Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A (September 2007). "Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis". Antimicrob. Agents Chemother. 51 (9): 3367–70. doi:10.1128/AAC.00041-07. PMC 2043228. PMID 17576830.
- ↑ Swarup V, Ghosh J, Mishra MK, Basu A (March 2008). "Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan". J. Antimicrob. Chemother. 61 (3): 679–88. doi:10.1093/jac/dkm503. PMID 18230688.
- ↑ Kazłowski B, Chiu YH, Kazłowska K, Pan CL, Wu CJ (August 2012). "Prevention of Japanese encephalitis virus infections by low-degree-polymerisation sulfated saccharides from Gracilaria sp. and Monostroma nitidum". Food Chem. 133 (3): 866–74. doi:10.1016/j.foodchem.2012.01.106.
- ↑ Dutta K, Ghosh D, Basu A (May 2009). "Curcumin Protects Neuronal Cells from Japanese Encephalitis Virus-Mediated Cell Death and also Inhibits Infective Viral Particle Formation by Dysregulation of Ubiquitin-Proteasome System". J Neuroimmune Pharmacol. 4 (3): 328–37. doi:10.1007/s11481-009-9158-2. PMID 19434500. S2CID 24691000. Archived from the original on 28 August 2021. Retrieved 31 January 2020.
- ↑ Mishra MK, Basu A (June 2008). "Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis". J. Neurochem. 105 (5): 1582–95. doi:10.1111/j.1471-4159.2008.05238.x. PMID 18208541.
- ↑ Mishra MK, Dutta K, Saheb SK, Basu A (December 2009). "Understanding the molecular mechanism of blood–brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent". Neurochem Int. 55 (8): 717–23. doi:10.1016/j.neuint.2009.07.006. PMID 19628016. S2CID 26964251. Archived from the original on 28 August 2021. Retrieved 31 January 2020.
External links
- Centers for Disease Control and Prevention Questions and Answers About Japanese Encephalitis Archived 1 October 2017 at the Wayback Machine
- Australian government Department of Health and Aging, Japanese Encephalitis Archived 21 March 2012 at the Wayback Machine, 2012
- CDC Japanese Encephalitis Surveillance and Immunization — Asia and Western Pacific Regions, 2016 Archived 19 September 2020 at the Wayback Machine, MMWR, June 9, 2017, 66(22);579–583
| Classification | |
|---|---|
| External resources |