Aldicarb
 | |
 | |
| Names | |
|---|---|
| IUPAC name
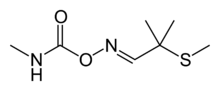
2-Methyl-2-(methylthio)propanal O-(N-methylcarbamoyl)oxime | |
| Other names
Temik | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.749 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C7H14N2O2S |
| Molar mass | 190.26 g·mol−1 |
| Appearance | colorless crystals |
| Odor | faint sulfur odor |
| Density | 1.195 g/cm2 |
| Melting point | 99.5 °C (211.1 °F; 372.6 K) |
| Boiling point | 251 °C (484 °F; 524 K) |
Solubility in water |
0.573 g/100 mL |
| Solubility | soluble in acetone, benzene, chlorobenzene, ethyl ether, isopropane, methylene chloride, toluene slightly soluble in xylene |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
0.84 mg/kg (oral, rats)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide.[2] Aldicarb is a cholinesterase inhibitor which prevents the breakdown of acetylcholine in the synapse. In case of severe poisoning, the victim dies of respiratory failure.
Aldicarb was first synthesized in 1965 by Payne and Weiden, and was sold on the market for the first time in 1970. [3] The synthesis of aldicarb results in both the E and Z isomers. [4]
Aldicarb is one of the most widely used pesticides internationally, and is also one of the most environmentally toxic. Aldicarb poisoning from agricultural water runoff has led to the destruction of healthy ecosystems and the irreversible poisoning of fertile agricultural land. Aldicarb is effective where resistance to organophosphate insecticides has developed, and is extremely important in potato production, where it is used for the control of soil-borne nematodes and some foliar pests. Its high level of solubility restricts its use in certain areas where the water table is close to the surface.
Regulatory status
In the United States, aldicarb was approved by the EPA for use by professional pesticide applicators on a variety of crops, including cotton, beans, and others. It is not approved for household use.[5] The EPA started limiting the main aldicarb pesticide, Temik 15G, in 2010, requiring an end to distribution by 2017. Discontinuation of the use on citrus and potatoes began in 2012, with a complete phase out of the product expected by 2018.[6] A new aldicarb pesticide named AgLogic 15G, was approved by the EPA in December 2011 and is said to be entering the market in 2015.[7] It will be registered for use on cotton, dry beans, peanuts, soybeans, sugar beets, and sweet potatoes.
Tres Pasitos, a mouse, rat, and roach killer that contains high concentrations of aldicarb, has been illegally imported into the United States from Mexico and other Latin American countries. The product is highly toxic to animals and people, and according to the EPA "should never be used in [the] home."[8]
Most commonly, aldicarb causes toxic symptoms when it is ingested through food that has been tainted with the insecticide.[9] Once in the body, aldicarb is broken down into aldicarb sulfone and aldicarb sulfoxide. Hydrolysis of aldicarb leads to aldicarb oximes and aldicarb nitriles to reverse the toxicity. [10]
Aldicarb is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[11]
History
Aldicarb is manufactured by Bayer CropScience, but was formerly owned and produced by Union Carbide. Union Carbide's agricultural chemicals division was sold to Rhône-Poulenc. Later, Aventis Cropscience was formed from Hoechst AG and Rhone-Poulenc Agrochemical, which lasted until Bayer acquired it in 2002.
In August 1979, groundwater wells in Suffolk County, NY were contaminated with aldicarb residue due to irrigated potato fields nearby. Of the approximately 8,400 wells tested, 13.5% contained more than 7 ug/l of aldicarb, which exceeds standard guidelines.[12]
In July 1985, aldicarb present in watermelons grown in California caused an outbreak of pesticide food poisoning which affected over 2,000 people, and led to a temporary ban on watermelon sales.[6]
In November 2009, corn treated with Temik was placed in and around peanut fields in Eastland County, Texas, near the town of Cisco. The corn was eaten by feral hogs, deer, and other animals, prompting the Texas Parks and Wildlife Department to issue a hunting ban.[13]
Toxicity in mammals
Aldicarb is a fast-acting cholinesterase inhibitor, causing rapid accumulation of acetylcholine at the synaptic cleft. The aldicarb structure is similar to that of acetylcholine, therefore improving its binding to acetylcholinesterase in the body. [14] It is widely used to study cholinergic neurotransmission in simple systems such as the nematode C. elegans.
Exposure to high amounts of aldicarb can cause weakness, blurred vision, headache, nausea, tearing, sweating, and tremors in humans. High doses can be fatal to humans because it can paralyze the respiratory system.[8]
In South Africa (where Aldicarb is popularly known as Two Step) it is widely used by burglars to poison dogs.[15][16][17]
References
- ↑ Kök, Fatma N; Arıca, M Yakup; Gencer, Oktay; Abak, Kazım; Hasırcı, Vasıf (1999). "Controlled release of aldicarb from carboxymethyl cellulose microspheres: in vitro and field applications". Pesticide Science. 55 (12): 1194–1202. doi:10.1002/(sici)1096-9063(199912)55:12<1194::aid-ps79>3.0.co;2-h.
- ↑ "Temik". www.bayercropscienceus.us. Retrieved 2011-04-01.
- ↑ Payne Jr., L. K.; Stansbury Jr., H. A.; Weiden, M. H. J. (July 1, 1966). "Synthesis and Insecticidal Properties of Some Cholinergic Trisubstituted Acetaldehyde O-(Methylcarbamoyl)oximes". Journal of Agricultural and Food Chemistry. 14 (4): 356-365. doi:10.1021/jf60146a007. Retrieved November 7, 2021.
- ↑ "PubChem - Aldicarb (CID: 9570071)". PubChem. National Library of Medicine. Retrieved November 7, 2021.
- ↑ "Aldicarb". extoxnet.orst.edu. The Extension Toxicology Network. June 1996. Retrieved 2007-08-13.
- 1 2 "Toxic Pesticide Banned after Decades of Use". Scientific American. August 18, 2010. Retrieved 2012-12-03.
- ↑ "AgLogic/Meymik 15 G Homepage". AgLogic. AgLogic Chemical. 2014. Retrieved 2015-10-02.
- 1 2 Avoid Illegal Household Pesticide Products, US Environmental Protection Agency
- ↑ "PubChem - Aldicarb (CID: 9570071)". PubChem. National Library of Medicine. Retrieved November 7, 2021.
- ↑ "PubChem - Aldicarb (CID: 0570071)". PubChem. Retrieved November 7, 2021.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ International Agency for Research on Cancer (1991). Occupational Exposures in Insecticide Application, and Some Pesticides (PDF). Lyon, France: World Health Organization. p. 93-113. Retrieved November 7, 2021.
- ↑ Authorities Investigate Contaminated Corn in Eastland County, Texas Parks and Wildlife Department, Nov. 5, 2009
- ↑ Payne Jr., L. K.; Stansbury Jr., H. A.; Weiden, M. H. J. (July 1, 1966). "Synthesis and Insecticidal Properties of Some Cholinergic Trisubstituted Acetaldehyde O-(Methylcarbamoyl)oximes". Journal of Agricultural and Food Chemistry. 14 (4): 356-365. doi:10.1021/jf60146a007. Retrieved November 7, 2021.
- ↑ Criminals target dogs with poison, IOL News, June 11, 2006
- ↑ Dog poisoning with the intention to break into houses, South Africa Today, July 10, 2014
- ↑ "Dog-poisoning plague hits city". News24. Retrieved 2017-04-21.
External links
- Aldicarb in the Pesticide Properties DataBase (PPDB)