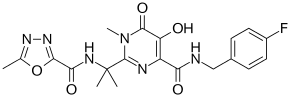
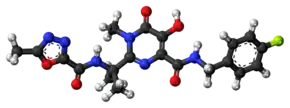
Raltegravir
 | |
 | |
| Names | |
|---|---|
| Trade names | Isentress |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | oral |
| Defined daily dose | 0.8 gram[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608004 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 60%(FDA) |
| Protein binding | 83% |
| Metabolism | Hepatic (UGT1A1) |
| Elimination half-life | 9 hours |
| Excretion | feces and urine |
| Chemical and physical data | |
| Formula | C20H21FN6O5 |
| Molar mass | 444.423 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Raltegravir (RAL), sold under the brand name Isentress, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS.[2] It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure.[3] It is taken by mouth.[2]
Common side effects include trouble sleeping, feeling tired, nausea, high blood sugar, and headaches.[3] Severe side effects may include allergic reactions including Stevens–Johnson syndrome, muscle breakdown, and liver problems.[3] It is unclear if use during pregnancy or breastfeeding is safe.[3] Raltegravir is an HIV integrase strand transfer inhibitor which blocks the functioning of HIV integrase which is needed for viral replication.[3]
Raltegravir was approved for medical use in the United States in 2007.[3] It is on the World Health Organization's List of Essential Medicines.[4] In the United Kingdom it costs the NHS about 472 pounds per month.[2] Lamivudine/raltegravir, a combination with lamivudine, is also available.[3]
Medical uses
Raltegravir was initially approved only for use in individuals whose infection has proven resistant to other HAART drugs.[5] However, in July 2009, the FDA granted expanded approval for raltegravir for use in all patients.[6] As with any HAART medication, raltegravir is unlikely to show durability if used as monotherapy, due to the highly mutagenic nature of HIV. Raltegravir is taken orally twice daily.[5]
In December 2011, it approval for use in children over the age of two, taken in pill form orally twice a day by prescription with two other antiretroviral medications to form the cocktail (most anti-HIV drugs regimens for adults and children use these cocktails). Raltegravir is available in chewable form, but because the two tablet formulations are not interchangeable, the chewable pills are only approved for use in children two to 11. Older adolescents will use the adult formulation.[7]
Efficacy
In a study of the drug as part of combination therapy, raltegravir exhibited potent and durable antiretroviral activity similar to that of efavirenz at 24 and 48 weeks but achieved HIV-1 RNA levels below detection at a more rapid rate. After 24 and 48 weeks of treatment, raltegravir did not result in increased serum levels of total cholesterol, low-density lipoprotein cholesterol, or triglycerides.[8][9]
Dosage
The defined daily dose is 0.8 gram (by mouth).[1]
Side effects
Raltegravir was generally well tolerated when used in combination with optimized background therapy regimens in treatment-experienced patients with HIV-1 infection in trials of up to 48 weeks' duration.[10]
Mechanism of action
As an integrase inhibitor, raltegravir targets integrase, an HIV enzyme that integrates the viral genetic material into human chromosomes, a critical step in the pathogenesis of HIV. The drug is metabolized away via glucuronidation.[11]
History
Raltegravir was the first integrase inhibitor to receive approval in the United States.[12][5][13]
Research
Raltegravir significantly alters HIV viral dynamics and decay and further research in this area is ongoing. In clinical trials patients taking raltegravir achieved viral loads less than 50 copies per millitre sooner than those taking similarly potent non-nucleoside reverse transcriptase inhibitors or protease inhibitors. This statistically significant difference in viral load reduction has caused some HIV researchers to begin questioning long held paradigms about HIV viral dynamics and decay.[14] Research into raltegravir's ability to affect latent viral reservoirs and possibly aid in the eradication of HIV is currently ongoing.[15]
Research results were published in the New England Journal of Medicine on July 24, 2008. The authors concluded that "raltegravir plus optimized background therapy provided better viral suppression than optimized background therapy alone for at least 48 weeks." [16]
Research on human cytomegalovirus (HCMV) terminase proteins demonstrated that raltegravir may block viral replication of the herpesviruses.[17]
In January 2013, a Phase II trial was initiated to evaluate the therapeutic benefit of raltegravir in treating multiple sclerosis (MS).[18] The drug is active against Human Endogenous Retroviruses (HERVs) and possibly Epstein–Barr virus, which have been suggested in the pathogenesis of relapsing-remitting MS.
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 24 September 2020. Retrieved 11 September 2020.
- 1 2 3 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 429. ISBN 9780857111562.
- 1 2 3 4 5 6 7 "Raltegravir Potassium". The American Society of Health-System Pharmacists. Archived from the original on 11 December 2017. Retrieved 8 December 2017.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 "Isentress Drug Approval Package". U.S. Food and Drug Administration (FDA). February 22, 2008. Archived from the original on 2012-10-17. Retrieved 2009-11-15.
- ↑ "UPDATE 2-FDA OKs widened use of Merck's Isentress HIV drug". Reuters. 2009-07-10. Archived from the original on 2021-05-14. Retrieved 2017-07-01.
- ↑ "FDA Okays Raltegravir for Kids, Teens with HIV". Archived from the original on 2013-12-19. Retrieved 2011-12-23.
- ↑ Markowitz M, Nguyen BY, Gotuzzo E, et al. (2007). "Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study". J. Acquir. Immune Defic. Syndr. 46 (2): 125–33. doi:10.1097/QAI.0b013e318157131c. PMID 17721395.
- ↑ Stephenson J (2007). "Researchers buoyed by novel HIV drugs: will expand drug arsenal against resistant virus". JAMA. 297 (14): 1535–6. doi:10.1001/jama.297.14.1535. PMID 17426263.
- ↑ Croxtall JD, Keam SJ (2009). "Raltegravir". Drugs. 69 (8): 1059–75. doi:10.2165/00003495-200969080-00007. PMID 19496631. Archived from the original on 2011-10-08. Retrieved 2010-03-30.
- ↑ "HIV Antiretroviral Agents in Development". www.thebody.com. Archived from the original on 2020-06-04. Retrieved 2020-03-31.
- ↑ "FDA approval of Isentress (raltegravir)". U.S. Food and Drug Administration (FDA). June 25, 2009. Archived from the original on 2009-07-10. Retrieved 2009-11-15.
- ↑ Durrant, Jacob D.; McCammon, J. Andrew (October 28, 2011). "Molecular dynamics simulations and drug discovery". BMC Biology. 9 (1): 71. doi:10.1186/1741-7007-9-71. PMC 3203851. PMID 22035460. Archived from the original on 2021-08-29. Retrieved 2020-03-31 – via BioMed Central.
- ↑ "Faster Viral Decay With Raltegravir". www.thebodypro.com. Archived from the original on 2020-08-06. Retrieved 2020-03-31.
- ↑ Clinical trial number NCT00554398 for "Impact of MK-0518 (Raltegravir) Intensification on HIV-1 Viral Latency in Patients With Previous Complete Viral Suppression" at ClinicalTrials.gov
- ↑ Steigbigel RT, Cooper DA, Kumar PN, et al. (July 2008). "Raltegravir with optimized background therapy for resistant HIV-1 infection". N. Engl. J. Med. 359 (4): 339–54. doi:10.1056/NEJMoa0708975. PMID 18650512.
- ↑ "Drug against AIDS could be effective against herpesvirus". ScienceDaily. Archived from the original on 2016-03-05. Retrieved 2018-03-09.
- ↑ "Raltegravir (Isentress) Pilot Study in Relapsing Multiple Sclerosis - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 2020-08-06. Retrieved 2020-03-31.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|
- MK-0518 at Aidsmedscom
- Integrase Inhibitor Raltegravir (MK-0518) Doubles HIV Suppression in Treatment-Experienced Patients Archived 2008-07-06 at the Wayback Machine (aidsmap 28 February 2007)
- RMK-0518 Abstract from CROI 2007
- World patent covering the potassium salt Archived 2007-10-14 at the Wayback Machine
- Raltegravir Pharmacokinetics Archived 2011-07-27 at the Wayback Machine