Seltorexant
 | |
| Clinical data | |
|---|---|
| Other names | MIN-202; JNJ-42847922; JNJ-922 |
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 2–3 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
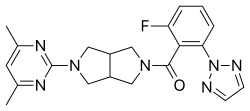
| Formula | C21H22FN7O |
| Molar mass | 407.453 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Seltorexant (former developmental code names MIN-202, JNJ-42847922, JNJ-922) is a selective, small-molecule antagonist of the OX2 receptor that is under development by Minerva Neurosciences and Johnson & Johnson's Janssen Pharmaceuticals for the treatment of insomnia and major depressive disorder (MDD).[2][3][4] As of December 2015, it is in phase II clinical trials for both insomnia and major depressive disorder.[3][5][6]
See also
References
- ↑ Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J (November 2020). "Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders". Expert Opin Drug Metab Toxicol. 16 (11): 1063–1078. doi:10.1080/17425255.2020.1817380. PMID 32901578. S2CID 221572078.
- ↑ Christopher JA (2014). "Small-molecule antagonists of the orexin receptors". Pharmaceutical Patent Analyst. 3 (6): 625–38. doi:10.4155/ppa.14.46. PMID 25489915.
- 1 2 Zisapel N (March 2015). "Current Phase II investigational therapies for insomnia". Expert Opinion on Investigational Drugs. 24 (3): 401–11. doi:10.1517/13543784.2015.987340. PMID 25423562. S2CID 8202006.
- ↑ Boss C, Roch C (August 2015). "Recent trends in orexin research--2010 to 2015". Bioorganic & Medicinal Chemistry Letters. 25 (15): 2875–87. doi:10.1016/j.bmcl.2015.05.012. PMID 26045032.
- ↑ "JNJ 42847922". AdisInsight. Retrieved 2015-05-19.
- ↑ Medicines in Development for Mental Health (PDF) (Report). Pharmaceutical Research and Manufacturers of America. 2014. Retrieved 2015-05-19.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.