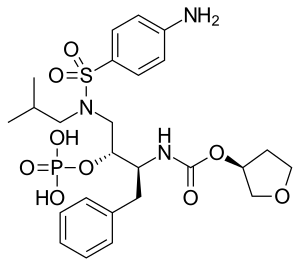
Fosamprenavir
 | |
 | |
| Names | |
|---|---|
| Trade names | Lexiva, Telzir |
IUPAC name
| |
| Clinical data | |
| Drug class | Protease inhibitor[1] |
| Main uses | Treat and prevent HIV/AIDS[1] |
| Side effects | Diarrhea, vomiting, headache, rash[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 700 mg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604012 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Unknown |
| Protein binding | 90% |
| Metabolism | Hydrolysed to amprenavir and phosphate in GI tract epithelium |
| Elimination half-life | 7.7 hours |
| Excretion | Fecal (as metabolites of amprenavir) |
| Chemical and physical data | |
| Formula | C25H36N3O9PS |
| Molar mass | 585.608 g/mol 623.700 g/mol (calcium salt) g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fosamprenavir, sold under the brand name Lexiva and Telzir, is a medication used to treat and prevent HIV/AIDS.[1] It is used together with other medications, including typically a low amount of ritonavir.[1][2] It is taken by mouth.[1]
Common side effects include diarrhea, vomiting, headache, and rash.[1] Other side effects may include Stevens-Johnson syndrome, sulfonamide allergy, high blood sugar, immune reconstitution syndrome, abnormal lipids, and kidney stones.[1] While it has been used in pregnancy, safety is unclear.[3] It is a protease inhibitor and pro-drug of amprenavir.[1][2]
Fosamprenavir was approved for medical use in the United States in 2003 and Europe in 2004.[1][4] It is available as a generic medication.[5] In the United Kingdom a month of medication costs the NHS about £260 as of 2021.[2] In the United States this amount costs about 300 USD.[5]
Medical uses
Fosamprenavir is used for the treatment of HIV-1 infections, typically but not necessarily in combination with low-dose ritonavir or other antiviral drugs.[6][7]
Dosage
It is used at a dose of 700 mg twice per day.[2]
Side effects
The most common side effect is diarrhea. Other common side effects include headache, dizziness and exanthema, which is usually transient. Severe allergic reactions (Stevens–Johnson syndrome) are rare.[6]
A direct comparison with lopinavir showed the two drugs to have comparable potency, but people on fosamprenavir tended to have a higher serum cholesterol.[8] Fosamprenavir's main advantage over lopinavir is that it is cheaper.
Interactions
Amprenavir (the active metabolite of fosamrenavir, which is found in blood plasma, liver and other organs) is metabolized via the liver enzyme CYP3A4 and also weakly inhibits this enzyme. This means that combination with drugs that are also metabolized by CYP3A4 can increase their plasma concentrations and thus side effects; and combination with drugs that inhibit CYP3A4 can increase amprenavir concentrations.[6]
When combining fosamprenavir with low doses of the CYP3A4 inhibitor ritonavir, this interaction is intended as it allows for application of lower fosamprenavir doses.[6]
Pharmacology
Fosamprenavir is quickly activated to amprenavir, even before it reaches the circulation. Amprenavir is a HIV protease inhibitor.[6]
References
- 1 2 3 4 5 6 7 8 9 10 "Fosamprenavir Monograph for Professionals". Drugs.com. Archived from the original on 28 June 2021. Retrieved 13 December 2021.
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 697. ISBN 978-0857114105.
- ↑ "Fosamprenavir Use During Pregnancy". Drugs.com. Archived from the original on 27 June 2021. Retrieved 13 December 2021.
- ↑ "Telzir". Archived from the original on 15 May 2021. Retrieved 13 December 2021.
- 1 2 "Fosamprenavir Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 13 December 2021.
- 1 2 3 4 5 Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 8009–17. ISBN 978-3-85200-181-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Lexiva. Drugs.com (Report). Monograph.
- ↑ Eron J, Yeni P, Gathe J, Estrada V, DeJesus E, Staszewski S, et al. (August 2006). "The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial". Lancet. 368 (9534): 476–82. doi:10.1016/S0140-6736(06)69155-1. PMID 16890834. S2CID 33612672.
- ↑ Shen CH, Wang YF, Kovalevsky AY, Harrison RW, Weber IT (September 2010). "Amprenavir complexes with HIV-1 protease and its drug-resistant mutants altering hydrophobic clusters". The FEBS Journal. 277 (18): 3699–714. doi:10.1111/j.1742-4658.2010.07771.x. PMC 2975871. PMID 20695887.
External links
- "Fosamprenavir". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-02-25. Retrieved 2021-03-18.
| Identifiers: |
|
|---|