Hepatitis E
| Hepatitis E | |
|---|---|
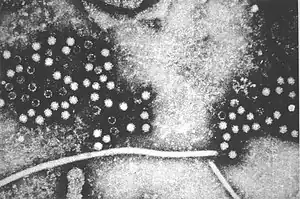 | |
| Hepatitis E virus | |
| Specialty | Infectious disease, gastroenterology |
| Symptoms | Tiredness, nausea, pain over liver, jaundice[1] |
| Complications | Cirrhosis, acute liver failure[1] |
| Usual onset | 2 to 10 wks after exposure[2] |
| Duration | 2 to 6 wks[2] |
| Causes | Hepatitis E virus (HEV)[1] |
| Diagnostic method | Blood test[1] |
| Differential diagnosis | Other types of viral hepatitis, leptospirosis, dengue, malaria[2][3] |
| Prevention | Clean water, toilets, vaccination[2] |
| Treatment | Symptomatic care, ribavirin (if chronic)[1] |
| Frequency | 20 million cases per year[2] |
| Deaths | 44,000 (2015)[2] |
Hepatitis E is an infection of the liver by the hepatitis E virus (HEV); it is a type of viral hepatitis.[2][1] Symptoms may include tiredness, nausea, pain over the liver, dark urine, and yellowish skin; though many have no symptoms.[1] Onset of symptoms is generally 2 to 10 weeks after exposure and symptoms last 2 to 6 weeks.[2] Occasionally, in people with a weakened immune system, the infection does not resolve and may result in complications such as cirrhosis.[1] Severe infections, resulting in acute liver failure, may occur in pregnant women.[1]
Hepatitis E is mainly spread by drinking contaminated water; though it may also spread by eating under-cooked meat.[2][1] It does not typically spread directly between people.[1] The virus is a positive-sense, nonenveloped, single-stranded, RNA icosahedral.[3] Diagnosis can be by blood or stool tests.[1] The disease can be separated into 8 genotypes, of which 4 primarily cause disease in people.[4] Other types of viral hepatitis include A, B, C, D, and X.[5]
Prevention is by using clean water and appropriate toilets.[2] A vaccine (HEV 239) is available to prevent the disease in China.[2][6] Treatment is generally symptomatic care including rest and drinking sufficient fluids.[1] Ribavirin may be used for chronic cases.[1] In 2015 it results in about 44,000 deaths.[2] During the third trimester of pregnancy up to 25% of women may die.[2]
In 2017, hepatitis E was estimated to affect more than 19 million people.[7] It occurs more commonly in the developing world, particularly in Asia.[1][2] Males aged 15 to 30 are most commonly affected.[8] It may occur as an outbreak due to poor access to safe water.[2] Outbreaks of hepatitis E date back to at least 1955 in New Delhi.[9] The virus was isolated in 1983 by Russian scientists investigating an outbreak in Afghanistan.[8]
Signs and symptoms
Acute infection
The average incubation period of hepatitis E is 40 days, ranging from 2 to 8 weeks. After a short prodromal phase symptoms may include jaundice, fatigue, and nausea, though most HEV infections are asymptomatic. The symptomatic phase coincides with elevated hepatic aminotransferase levels.[10][11][12][13] Viral RNA becomes detectable in stool and blood serum during the incubation period. Serum IgM and IgG antibodies against HEV appear just before the onset of clinical symptoms. Recovery leads to virus clearance from the blood, while the virus may persist in stool for much longer. Recovery is also marked by disappearance of IgM antibodies and increase of levels of IgG antibodies.[2][12]
Chronic infection
While usually lasting weeks and then resolving, in people with weakened immune systems—particularly in people who have had solid organ transplant—hepatitis E may cause a chronic infection.[14] Occasionally this may result in a life-threatening illness such as fulminant liver failure or liver cirrhosis.[15][16]
Other organs
Infection with hepatitis E virus can also lead to problems in other organs. For some of these reported conditions such as musculoskeletal or immune-mediated manifestations the relationship is not entirely clear, but for several neurological and blood conditions the relationship appears more consistent:[17][18][19][20]
- Acute pancreatitis (HEV genotype 1[8])
- Neurological complications (though the mechanism of neurological damage is unknown at this point.[8]) include: Guillain-Barré syndrome (acute limb weakness due to nerve involvement), neuralgic amyotrophy (arm and shoulder weakness, also known as Parsonage-Turner syndrome), acute transverse myelitis and acute meningoencephalitis.
- Glomerulonephritis with nephrotic syndrome and/or cryoglobulinemia
- Mixed cryoglobulinemia, where antibodies in the bloodstream react inappropriately at low temperatures
- Severe thrombocytopenia (low platelet count in the blood) which confers an increased risk of dangerous bleeding
In pregnancy
Pregnant women show a more severe course of infection than other populations. Liver failure with mortality rates of 20% to 25% has been reported from outbreaks of genotype 1 and 2 HEV in developing countries. Besides signs of an acute infections, adverse effects on the mother and fetus may include preterm delivery, abortion, stillbirth, and neonatal death.[21][22][23]
The pathological and biological mechanisms behind the adverse outcomes of pregnancy infections remain largely unclear. Increased viral replication and influence of hormonal changes on the immune system are currently thought to contribute to worsening the course of infection.[24] Furthermore, studies showing evidence for viral replication in the placenta or reporting the full viral life cycle in placental-derived cells in vitro suggest that the human placenta may be a site of viral replication outside the liver.[25] The primary reason for HEV severity in pregnancy remains enigmatic.[8]
Virology
Classification
HEV is classified into the family Hepeviridae, which is divided in two genera, Orthohepevirus (all mammalian and avian HEV isolates) and Piscihepevirus (cutthroat trout HEV).[24] Only one serotype of the virus is known, and classification is based on the nucleotide sequences of the genome.[26] Genotype 1 can be further subclassified into five subtypes,[27] genotype 2 into two subtypes,[28] and genotypes 3 and 4 have been divided into 10[29] and seven subtypes.[29] Additionally there are genotypes 5, 6, 7 and 8.[30] Rat HEV was first isolated from Norway rats in Germany,[31] and a 2018 CDC article indicated the detection of rat HEV RNA in a transplant recipient.[32]
Distribution
- Genotype 1 has been isolated from tropical and several subtropical countries in Asia and Africa.[33]
- Genotype 2 has been isolated from Mexico, Nigeria, and Chad.[34]
- Genotype 3 has been isolated almost worldwide including Asia, Europe, Oceania, and North and South America.[35]
- Genotype 4 appears to be limited to Asia and indigenous cases from Europe.[33][36]
Genotypes 1 and 2 are restricted to humans and often associated with large outbreaks and epidemics in developing countries with poor sanitation conditions.[33] Genotypes 3 and 4 infect humans, pigs, and other animal species and have been responsible for sporadic cases of hepatitis E in both developing and industrialized countries.[37][38]
Transmission
Hepatitis E (genotype 1 and, to a lesser extent genotype 2) is endemic and can cause outbreaks in Southeast Asia, northern and central Africa, India, and Central America.[8][39] It is spread mainly by the fecal–oral route due to contamination of water supplies or food; direct person-to-person transmission is uncommon.[8][11] In contrast to genotypes 1 and 2, genotypes 3 and 4 cause sporadic cases thought to be contracted zoonotically, from direct contact with animals or indirectly from contaminated water or undercooked meat.[8][40]
Outbreaks of epidemic hepatitis E most commonly occur after heavy rainfalls, especially monsoons because of their disruption of water supplies; heavy flooding can causes sewage to contaminate water supplies.[41][42]: 78 The World Health Organization recommendation for chlorine on HEV inactivation, a free chlorine residual of 0.5 mg/L (6.7×10−5 oz/US gal) for 30 min (pH, <8.0)[43] Major outbreaks have occurred in New Delhi, India (30,000 cases in 1955–1956),[44] Burma (20,000 cases in 1976–1977),[45] Kashmir, India (52,000 cases in 1978),[46] Kanpur, India (79,000 cases in 1991),[44] and China (100,000 cases between 1986 and 1988).[47] According to Rein et al., HEV genotypes 1 and 2 caused some 20.1 million hepatitis E infections, along with 3.4 million cases of symptomatic disease, and 70,000 deaths in 2005; however the aforementioned paper did not estimate the burden of genotypes 3 and 4.[48]
According to the Department for Environment, Food and Rural Affairs, evidence indicated the increase in hepatitis E in the U.K. was due to food-borne zoonoses, citing a study that found in the U.K. that 10% of pork sausages contained the hepatitis E virus. Some research suggests that food must reach a temperature of 70 °C (158 °F) for 20 minutes to eliminate the risk of infection. The Animal Health and Veterinary Laboratories Agency discovered hepatitis E in almost half of all pigs in Scotland.[49][50]
Hepatitis E infection appeared to be more common in people on hemodialysis, although the specific risk factors for transmission are not clear.[51]
Animal reservoir
Hepatitis E due to genotypes other than 1 and 2 is thought to be a zoonosis, in that animals are thought to be the primary reservoir; deer and swine have frequently been implicated.[52] Domestic animals have been reported as a reservoir for the hepatitis E virus, with some surveys showing infection rates exceeding 95% among domestic pigs.[53] Replicative virus has been found in the small intestine, lymph nodes, colon, and liver of experimentally infected pigs. Transmission after consumption of wild boar meat and uncooked deer meat has been reported as well.[54] The rate of transmission to humans by this route and the public health importance of this are, however, still unclear.[55] Other animal reservoirs are possible but unknown at this time[8]
A number of other small mammals have been identified as potential reservoirs: the lesser bandicoot rat (Bandicota bengalensis), the black rat (Rattus rattus brunneusculus) and the Asian house shrew (Suncus murinus). A new virus designated rat hepatitis E virus has been isolated.[56]
Genomics
HEV has three open reading frames (ORFs) encoding two polyproteins (O1 and O2 protein). ORF2 encodes three capsid proteins whereas O1 encodes seven fragments involved in viral replication, among others.[57][58][59]
The smallest ORF of the HEV genome, ORF3 is translated from a subgenomic RNA into O3, a protein of 113–115 amino acids. ORF3 is proposed to play critical roles in immune evasion by HEV. Previous studies showed that ORF3 is bound to viral particles found in patient sera and produced in cell culture. Although in cultured cells ORF3 has not appeared essential for HEV RNA replication, viral assembly, or infection, it is required for particle release.[60]
Virus lifecycle
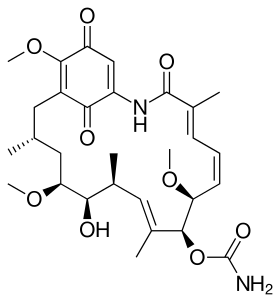
The lifecycle of hepatitis E virus is unknown; the capsid protein obtains viral entry by binding to a cellular receptor. ORF2 (c-terminal) moderates viral entry by binding to HSC70.[61][62]
Geldanamycin blocks the transport of HEV239 capsid protein, but not the binding/entry of the truncated capsid protein, which indicates that Hsp90 plays an important part in HEV transport.[61]
Diagnosis
In terms of the diagnosis of hepatitis E, only a laboratory blood test that confirms the presence of HEV RNA or IgM antibodies to HEV can be trusted.[63][64] In the United States no serologic tests for diagnosis of HEV infection have ever been authorized by the Food and Drug Administration.[63] The World Health Organization has developed an international standard strain for detection and quantification of HEV RNA.[65] In acute infection the viremic window for detection of HEV RNA closes 3 weeks after symptoms begin.[66]
Virological markers
Assuming that vaccination has not occurred, tests may show:[8]
- if the person's immune system is normal, then
- if IgM anti-HEV is negative, then there is no evidence of recent HEV infection
- if IgM anti-HEV is positive, then the person is likely to have a recent or current HEV infection
- if the person's immune system is weakened by disease or medical treatment, as in the case of a person who has received a solid organ transplant, then
- if IgM anti-HEV is negative, then if additional blood testing reveals
- positive HEV RNA then the person has HEV infection
- negative HEV RNA then there is no evidence of current or recent infection
- if IgM anti-HEV is positive, then the person is likely to have a recent or current HEV infection, and HEV RNA may be useful to track resolution
- if IgM anti-HEV is negative, then if additional blood testing reveals
Prevention
Sanitation
Sanitation is the most important measure in prevention of hepatitis E; this consists of proper treatment and disposal of human waste, higher standards for public water supplies, improved personal hygiene procedures, and sanitary food preparation. Thus, prevention strategies of this disease are similar to those of many other diseases that plague developing nations.[11] Cooking meat at 71 °C (159.8 °F) for five minutes kills the hepatitis E virus, different temperatures means different time to inactivate the virus.[50]
Blood products
The amount of virus present in blood products required to cause transfunction-transmitted infection (TTI) appears variable. Transfusion transmission of hepatitis E virus can be screened via minipool HEV NAT (Nucleic acid testing) screening.[67][68] NAT is a technique used to screen blood molecularly, when blood donations are received; it screens for TTI.[69]
Vaccines
A vaccine based on recombinant viral proteins was developed in the 1990s and tested in a high-risk population (in Nepal) in 2001.[70] The vaccine appeared to be effective and safe, but development was stopped for lack of profitability, since hepatitis E is rare in developed countries.[71] No hepatitis E vaccine is licensed for use in the United States.[63]
The exception is China; after more than a year of scrutiny and inspection by China's State Food and Drug Administration (SFDA), a hepatitis E vaccine developed by Chinese scientists was available at the end of 2012. The vaccine —called HEV 239 by its developer Xiamen Innovax Biotech— was approved for prevention of hepatitis E in 2012 by the Chinese Ministry of Science and Technology, following a controlled trial on 100,000+ people from Jiangsu Province where none of those vaccinated became infected during a 12-month period, compared to 15 in the group given placebo.[72] The first vaccine batches came out of Innovax' factory in late October 2012, to be sold to Chinese distributors.[71]
Due to lack of evidence, the World Health Organization has not made a recommendation regarding routine use of the HEV 239 vaccine as of 2015.[73] Its 2015 position was that national authorities may decide to use the vaccine based on their local epidemiology.[73]
Treatment
There is no drug that has established safety and effectiveness for hepatitis E, and there have been no large randomized clinical trials of antiviral drugs.[2] Reviews of existing small studies suggest that ribavirin can be considered effective in immunocompromised people who have developed chronic infection.[74][75]
Chronic HEV infection is associated with immunosuppressive therapies, and when that happens in individuals with solid-organ transplantation, reducing immunosuppressive medications can result in clearance of HEV in one third of patients.[8]
Epidemiology
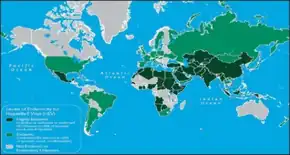
The hepatitis E virus causes around 20 million infections a year. These result in around three million acute illnesses and resulted in 44,000 deaths during 2015.[2] Pregnant women are particularly at risk of complications due to HEV infection, who can develop an acute form of the disease that is fatal in 30% of cases or more. HEV is a major cause of illness and of death in the developing world and disproportionate cause of deaths among pregnant women. Hepatitis E is endemic in Central Asia, while Central America and the Middle East have reported outbreaks.[76][77] Increasingly, hepatitis E is being seen in developed nations, with reports in 2015 of 848 cases of hepatitis E virus infection in England and Wales.[78]
Recent outbreaks
In October 2007, an epidemic of hepatitis E occurred in Kitgum District of northern Uganda. This outbreak progressed to become one of the largest known hepatitis E outbreaks in the world. By June 2009, it had resulted in illness in 10,196 persons and 160 deaths.[79] The aforementioned outbreak occurred despite no previous epidemics having been documented in the country, women were the most affected by HEV.[79]
In July 2012, an outbreak was reported in South Sudanese refugee camps in Maban County near the Sudan border. South Sudan's Ministry of Health reported over 400 cases and 16 fatalities as of 13 September 2012.[80] Progressing further, as of 2 February 2013, 88 died due to the outbreak. The medical charity Medecins Sans Frontieres said it treated almost 4000 people.[81] In April 2014, an outbreak in the Biratnagar Municipality of Nepal resulted in infection of over 6000 locals and at least 9 dead.[82]
During an outbreak in Namibia, the number of affected people rose from 490 in January 2018, to 5014 (with 42 deaths) by April 2019, to 6151 cases (with 56 deaths) by August 2019; the WHO estimated that the case fatality rate was 0.9%.[83][84][85]
In Hong Kong in May 2020, there were at least 10 cases of hepatitis E that were transmitted by rats, and possibly hundreds of cases that had a transmission mechanism that is not fully understood.[86]
Evolution
The strains of HEV that exist today may have arisen from a shared ancestor virus 536 to 1344 years ago.[87] Another analysis has dated the origin of Hepatitis E to ~6000 years ago, with a suggestion that this was associated with domestication of pigs.[88] At some point, two clades may have diverged — an anthropotropic form and an enzootic form — which subsequently evolved into genotypes 1 and 2 and genotypes 3 and 4, respectively.[89]
Whereas genotype 2 remains less commonly detected than other genotypes, genetic evolutionary analyses suggest that genotypes 1, 3, and 4 have spread substantially during the past 100 years.[8]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 "Hepatitis (Viral) NIDDK". The National Institute of Diabetes and Digestive and Kidney Diseases. Archived from the original on 2016-12-29. Retrieved 2020-06-19.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 "Hepatitis E". www.who.int. Archived from the original on 20 April 2021. Retrieved 22 May 2021.
- 1 2 Waqar, S; Sharma, B; Koirala, J (January 2021). "Hepatitis E". PMID 30335311.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Dalton, Harry R.; Kamar, Nassim; Baylis, Sally A.; Moradpour, Darius; Wedemeyer, Heiner; Negro, Francesco (June 2018). "EASL Clinical Practice Guidelines on hepatitis E virus infection". Journal of Hepatology. 68 (6): 1256–1271. doi:10.1016/j.jhep.2018.03.005. PMID 29609832. Archived from the original on 2021-08-28. Retrieved 2019-07-27.
- ↑ "What Is Viral Hepatitis?". National Institute of Diabetes and Digestive and Kidney Diseases. NIDDK. Archived from the original on 16 April 2021. Retrieved 23 May 2021.
- ↑ Li, Shao-Wei; Zhao, Qinjian; Wu, Ting; Chen, Shu; Zhang, Jun; Xia, Ning-Shao (2015-02-25). "The development of a recombinant hepatitis E vaccine HEV 239". Human Vaccines & Immunotherapeutics. 11 (4): 908–914. doi:10.1080/21645515.2015.1008870. ISSN 2164-5515. PMC 4514148. PMID 25714510.
- ↑ GBD 2017 Disease Injury Incidence Prevalence Collaborators (10 November 2018). "Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1789–1858. doi:10.1016/S0140-6736(18)32279-7. ISSN 1474-547X. PMC 6227754. PMID 30496104.
- 1 2 3 4 5 6 7 8 9 10 11 12 Izopet, Jacques; Abravanel, Florence; Dalton, Harry R.; Kamar, Nassim (1 January 2014). "Hepatitis E Virus Infection". Clinical Microbiology Reviews. 27 (1): 116–138. doi:10.1128/CMR.00057-13. ISSN 0893-8512. PMC 3910910. PMID 24396139.
- ↑ Kumar, Subrat; Subhadra, Subhra; Singh, Bhupinder; Panda, B.K. (April 2013). "Hepatitis E virus: the current scenario". International Journal of Infectious Diseases. 17 (4): e228–e233. doi:10.1016/j.ijid.2012.11.026. ISSN 1201-9712. PMID 23313154. Archived from the original on 2020-09-28. Retrieved 2019-07-27.
- ↑ Sanford, Christopher A.; Jong, Elaine C.; Pottinger, Paul S. (2016). The Travel and Tropical Medicine Manual E-Book. Elsevier Health Sciences. p. 324. ISBN 9780323417426. Archived from the original on 2021-05-28. Retrieved 2019-08-20.
- 1 2 3 "Hepatitis E Fact sheet". WHO. Archived from the original on 12 March 2016. Retrieved 17 April 2019.
- 1 2 Hoofnagle, J. H.; Nelson, K. E.; Purcell, R. H. (2012). "Hepatitis E". New England Journal of Medicine. 367 (13): 1237–1244. doi:10.1056/NEJMra1204512. PMID 23013075.
- ↑ "Facts about hepatitis E". ecdc.europa.eu. European Centre for Disease Prevention and Control. Archived from the original on 5 November 2021. Retrieved 17 April 2019.
- ↑ Bonnet, D.; Kamar, N.; Izopet, J.; Alric, L. (2012). "L'hépatite virale E : Une maladie émergente". La Revue de Médecine Interne. 33 (6): 328–334. doi:10.1016/j.revmed.2012.01.017. PMID 22405325.
- ↑ Behrendt, Patrick; Steinmann, Eike; Manns, Michael P.; Wedemeyer, Heiner (2014-12-01). "The impact of hepatitis E in the liver transplant setting". Journal of Hepatology. 61 (6): 1418–1429. doi:10.1016/j.jhep.2014.08.047. PMID 25195557.
- ↑ Kamar, Nassim; Pischke, Sven (7 May 2018). "Acute and Persistent Hepatitis E Virus Genotype 3 and 4 Infection: Clinical Features, Pathogenesis, and Treatment". Cold Spring Harbor Perspectives in Medicine. 9 (7): a031872. doi:10.1101/cshperspect.a031872. ISSN 2157-1422. PMC 6601456. PMID 29735575.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - ↑ Bazerbachi, F; Haffar, S; Garg, SK; Lake, JR (February 2016). "Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature". Gastroenterology Report. 4 (1): 1–15. doi:10.1093/gastro/gov042. PMC 4760069. PMID 26358655.
- ↑ Rivero-Juárez, Antonio; Aguilera, Antonio; Avellón, Ana; García-Deltoro, Miguel; García, Federico; Gortazar, Christian; Granados, Rafael; Macías, Juan; Merchante, Nicolás; Oteo, José Antonio; Pérez-Gracia, María Teresa; Pineda, Juan Antonio; Rivero, Antonio; Rodriguez-Lazaro, David; Téllez, Francisco; Morano-Amado, Luis E. (30 July 2018). "Executive summary: Consensus document of the diagnosis, management and prevention of infection with the hepatitis E virus: Study Group for Viral Hepatitis (GEHEP) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC)". Enfermedades Infecciosas y Microbiologia Clinica. 38 (1): 28–32. doi:10.1016/j.eimc.2018.06.014. ISSN 1578-1852. PMID 30072282.
- ↑ Kamar, Nassim; Bendall, Richard P.; Peron, Jean Marie; Cintas, Pascal; Prudhomme, Laurent; Mansuy, Jean Michel; Rostaing, Lionel; Keane, Frances; Ijaz, Samreen; Izopet, Jacques; Dalton, Harry R. (2011). "Hepatitis E virus and neurologic disorders". Emerging Infectious Diseases. 17 (2): 173–179. doi:10.3201/eid1702.100856. ISSN 1080-6059. PMC 3298379. PMID 21291585.
- ↑ Dalton, Harry R.; Kamar, Nassim; van Eijk, Jeroen J. J.; Mclean, Brendan N.; Cintas, Pascal; Bendall, Richard P.; Jacobs, Bart C. (29 December 2015). "Hepatitis E virus and neurological injury". Nature Reviews Neurology. 12 (2): 77–85. doi:10.1038/nrneurol.2015.234. ISSN 1759-4766. PMID 26711839. S2CID 25495184. Archived from the original on 29 August 2021. Retrieved 31 January 2020.
- ↑ Patra, Sharda; Kumar, Ashish; Trivedi, Shubha Sagar; Puri, Manju; Sarin, Shiv Kumar (2007-07-03). "Maternal and Fetal Outcomes in Pregnant Women with Acute Hepatitis E Virus Infection". Annals of Internal Medicine. 147 (1): 28–33. doi:10.7326/0003-4819-147-1-200707030-00005. ISSN 0003-4819. PMID 17606958. S2CID 44504380. Archived from the original on 2021-08-29. Retrieved 2020-01-31.
- ↑ Kumar A., Beniwal M., Kar P., Sharma J.B., Murthy N.S. (2004). "Wiley Online Library". International Journal of Gynecology & Obstetrics. 85 (3): 240–244. doi:10.1016/j.ijgo.2003.11.018. PMID 15145258. S2CID 72665101.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Khuroo, Mohammad S.; Khuroo, Mehnaaz S.; Khuroo, Naira S. (20 September 2016). "Transmission of Hepatitis E Virus in Developing Countries". Viruses. 8 (9): 253. doi:10.3390/v8090253. ISSN 1999-4915. PMC 5035967. PMID 27657112.
- 1 2 Pérez-Gracia, María Teresa; Suay-García, Beatriz; Mateos-Lindemann, María Luisa (20 March 2017). "Hepatitis E and pregnancy: current state". Reviews in Medical Virology. 27 (3): e1929. doi:10.1002/rmv.1929. ISSN 1099-1654. PMID 28318080. S2CID 44761962.
- ↑ Bose, Purabi Deka; Das, Bhudev Chandra; Hazam, Rajib Kishore; Kumar, Ashok; Medhi, Subhash; Kar, Premashis (2014). "Evidence of extrahepatic replication of hepatitis E virus in human placenta". Journal of General Virology. 95 (6): 1266–1271. doi:10.1099/vir.0.063602-0. PMID 24622580.
- ↑ Feldman, Mark; Friedman, Lawrence S.; Brandt, Lawrence J. (2010). Sleisenger and Fordtran's Gastrointestinal and Liver Disease E-Book: Pathophysiology, Diagnosis, Management, Expert Consult Premium Edition - Enhanced Online Features. Elsevier Health Sciences. p. 1337. ISBN 978-1-4377-2767-8. Archived from the original on 28 August 2021. Retrieved 27 June 2020.
- ↑ Wang, Youchun (2016). Hepatitis E Virus. Springer. p. 75. ISBN 9789402409420. Archived from the original on 2020-08-03. Retrieved 2019-08-22.
- ↑ Wang, Youchun (2016). Hepatitis E Virus. Springer. p. 10. ISBN 9789402409420. Archived from the original on 2020-11-19. Retrieved 2020-11-13.
- 1 2 Boyer, Thomas D.; Manns, Michael Peter; Sanyal, Arun J.; Zakim, David (2012). Zakim and Boyer's Hepatology: A Textbook of Liver Disease. Elsevier Health Sciences. p. 609. ISBN 978-1437708813. Archived from the original on 2020-08-03. Retrieved 2019-08-22.
- ↑ Sridhar, Siddharth; Teng, Jade L. L.; Chiu, Tsz-Ho; Lau, Susanna K. P.; Woo, Patrick C. Y. (20 April 2017). "Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants". International Journal of Molecular Sciences. 18 (4): 869. doi:10.3390/ijms18040869. ISSN 1422-0067. PMC 5412450. PMID 28425927.
- ↑ Johne, Reimar; Heckel, Gerald; Plenge-Bönig, Anita; Kindler, Eveline; Maresch, Christina; Reetz, Jochen; Schielke, Anika; Ulrich, Rainer G. (2010). "Novel hepatitis E virus genotype in Norway rats, Germany". Emerging Infectious Diseases. 16 (9): 1452–1455. doi:10.3201/eid1609.100444. ISSN 1080-6059. PMC 3294985. PMID 20735931.
- ↑ Sridhar, Siddharth; Yip, Cyril C.Y.; Wu, Shusheng; Cai, Jianpiao; Zhang, Anna Jin-Xia; Leung, Kit-Hang; Chung, Tom W.H.; Chan, Jasper F.W.; Chan, Wan-Mui; Teng, Jade L.L.; Au-Yeung, Rex K.H.; Cheng, Vincent C.C.; Chen, Honglin; Lau, Susanna K.P.; Woo, Patrick C.Y.; Xia, Ning-Shao; Lo, Chung-Mau; Yuen, Kwok-Yung (December 2018). "Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant". Emerging Infectious Diseases. 24 (12): 2241–2250. doi:10.3201/eid2412.180937. PMC 6256372. PMID 30457530.
- 1 2 3 Song, Yoon-Jae (2010-11-01). "Studies of hepatitis E virus genotypes". The Indian Journal of Medical Research. 132 (5): 487–488. ISSN 0971-5916. PMC 3028963. PMID 21149996.
- ↑ Pelosi, E; Clarke, I (2008-11-07). "Hepatitis E: a complex and global disease". Emerging Health Threats Journal. 1: e8. doi:10.3134/ehtj.08.008. ISSN 1752-8550. PMC 3167588. PMID 22460217.
- ↑ Hepatitis E Vaccine Working Group (1 October 2014). Recommendations of HEV Working Group on the use of hepatitis E vaccine (PDF). www.who.int (Report). WHO. Archived (PDF) from the original on 9 August 2020. Retrieved 5 October 2020.
- ↑ Guerrant, Richard L.; Walker, David H.; Weller, Peter F. (2011). Tropical Infectious Diseases: Principles, Pathogens and Practice (Expert Consult – Online and Print). Elsevier Health Sciences. p. 424. ISBN 9781437737776. Archived from the original on 2020-08-03. Retrieved 2019-08-22.
- ↑ Meng, X. J. (2010-01-27). "Hepatitis E virus: Animal Reservoirs and Zoonotic Risk". Veterinary Microbiology. 140 (3–4): 256–65. doi:10.1016/j.vetmic.2009.03.017. ISSN 0378-1135. PMC 2814965. PMID 19361937.
- ↑ Woolson, K. L.; Forbes, A.; Vine, L.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J. G.; Madden, R. G.; Glasgow, T.; Kotecha, A.; Dalton, H. C.; Mihailescu, L.; Warshow, U.; Hussaini, H. S.; Palmer, J.; Mclean, B. N.; Haywood, B.; Bendall, R. P.; Dalton, H. R. (2014). "Extra-hepatic manifestations of autochthonous hepatitis E infection". Alimentary Pharmacology & Therapeutics. 40 (11–12): 1282–1291. doi:10.1111/apt.12986. ISSN 1365-2036. PMID 25303615. S2CID 207050371.
- ↑ Liu, Dongyou (2010-11-23). Molecular Detection of Human Viral Pathogens. CRC Press. p. 102. ISBN 9781439812372. Archived from the original on 2018-02-15. Retrieved 2016-02-26.
- ↑ Dai, Xing; Dong, Chen; Zhou, Zhenxian; Liang, Jiuhong; Dong, Min; Yang, Yan; Fu, Jianguang; Tian, Hua; Wang, Song; Fan, Jie; Meng, Jihong; Purdy, Michael A. (2013). "Hepatitis E Virus Genotype 4, Nanjing, China, 2001–2011". Emerging Infectious Diseases. 19 (9): 1528–1530. doi:10.3201/eid1909.130013. ISSN 1080-6040. PMC 3810912. PMID 23965731.
- ↑ Kanki, Phyllis; Grimes, D. Jay (2012). Infectious Diseases: Selected Entries from the Encyclopedia of Sustainability Science and Technology. Springer Science & Business Media. p. 391. ISBN 9781461457190. Archived from the original on 2020-08-03. Retrieved 2019-08-17.
- ↑ Griffiths, Jeffrey; Maguire, James H.; Heggenhougen, Kristian; Quah, Stella R. (2010-03-09). Public Health and Infectious Diseases. Elsevier. ISBN 9780123815071. Archived from the original on 2019-05-28. Retrieved 2016-02-26.
- ↑ Guerin, Philippe Jean; Nicand, Elisabeth; Ciglenecki, Iza; Grais, Rebecca Freeman; Moren, Alain; Diaz, Francisco; Tatay, Mercedes; Nizou, Jacques-Yves; Pinoges, Loretxu; Hamid, Nuha; Boccia, Delia; Klovstad, Hilde; Guthmann, Jean-Paul (15 June 2006). "A Large Outbreak of Hepatitis E among a Displaced Population in Darfur, Sudan, 2004: The Role of Water Treatment Methods". Clinical Infectious Diseases. 42 (12): 1685–1691. doi:10.1086/504321. ISSN 1058-4838. PMID 16705572.
- 1 2 Bitton, Gabriel (2005-05-27). Wastewater Microbiology. John Wiley & Sons. p. 132. ISBN 9780471717911. Archived from the original on 2019-05-28. Retrieved 2016-02-26.
- ↑ Hubálek, Zdenek; Rudolf, Ivo (2010-11-25). Microbial Zoonoses and Sapronoses. Springer Science & Business Media. p. 189. ISBN 9789048196579. Archived from the original on 2020-08-03. Retrieved 2016-02-26.
- ↑ Khuroo, Mohammad Sultan (2011-10-01). "Discovery of hepatitis E: The epidemic non-A, non-B hepatitis 30 years down the memory lane". Virus Research. Hepatitis E Viruses. 161 (1): 3–14. doi:10.1016/j.virusres.2011.02.007. PMID 21320558.
- ↑ Cowie, Benjamin C.; Adamopoulos, Jim; Carter, Karen; Kelly, Heath (2005-03-01). "Hepatitis E Infections, Victoria, Australia". Emerging Infectious Diseases. 11 (3): 482–484. doi:10.3201/eid1103.040706. ISSN 1080-6040. PMC 3298235. PMID 15757573.
- ↑ Rein, David B.; Stevens, Gretchen A.; Theaker, Jordan; Wittenborn, John S.; Wiersma, Steven T. (2012). "The global burden of hepatitis E virus genotypes 1 and 2 in 2005". Hepatology. 55 (4): 988–997. doi:10.1002/hep.25505. ISSN 1527-3350. PMID 22121109. S2CID 25571762.
- ↑ Doward, Jamie (21 September 2013). "Chefs fight for the right to serve their pork pink". The Observer newspaper. Archived from the original on 25 February 2017. Retrieved 11 December 2016.
- 1 2 "Hepatitis E Virus and Food". www.fsai.ie. Food Safety Authority of Ireland. 11 July 2017. Archived from the original on 28 May 2019. Retrieved 27 July 2019.
- ↑ Haffar, Samir; Bazerbachi, Fateh (2017-09-04). "Systematic review with meta-analysis: the association between hepatitis E seroprevalence and haemodialysis". Aliment Pharmacol Ther. 46 (9): 790–799. doi:10.1111/apt.14285. PMID 28869287. S2CID 24480333. Archived from the original on 2021-08-28. Retrieved 2020-01-31.
- ↑ Pavio, Nicole; Meng, Xiang-Jin; Renou, Christophe (2010-01-01). "Zoonotic hepatitis E: animal reservoirs and emerging risks". Veterinary Research. 41 (6): 46. doi:10.1051/vetres/2010018. ISSN 0928-4249. PMC 2865210. PMID 20359452.
- ↑ Satou K, Nishiura H; Nishiura (2007). "Transmission Dynamics of Hepatitis E Among Swine: Potential Impact upon Human Infection". BMC Vet. Res. 3: 9. doi:10.1186/1746-6148-3-9. PMC 1885244. PMID 17493260.
- ↑ Li TC, Chijiwa K, Sera N (2005). "Hepatitis E Virus Transmission from Wild Boar Meat". Emerging Infect. Dis. 11 (12): 1958–60. doi:10.3201/eid1112.051041. PMC 3367655. PMID 16485490.
- ↑ Kuniholm MH & Nelson KE (2008). "Of Organ Meats and Hepatitis E Virus: One Part of a Larger Puzzle Is Solved". J Infect Dis. 198 (12): 1727–1728. doi:10.1086/593212. PMID 18983247.
- ↑ Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A; Plenge-Bönig; Hess; Ulrich; Reetz; Schielke (2010). "Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR". J. Gen. Virol. 91 (Pt 3): 750–758. doi:10.1099/vir.0.016584-0. PMID 19889929.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Balakrishnan, V.; Rajesh, G. (2016). Practical Gastroenterology. JP Medical Ltd. p. 195. ISBN 9789352501908. Archived from the original on 3 August 2020. Retrieved 22 July 2019.
- ↑ Cocquerel, Laurence; Dubuisson, Jean; Meuleman, Philip; d'Autume, Valentin de Masson; Farhat, Rayan; Aliouat-Denis, Cécile-Marie; Duvet, Sandrine; Saas, Laure; Wychowski, Czeslaw; Saliou, Jean-Michel; Sayed, Ibrahim M.; Montpellier, Claire; Ankavay, Maliki (18 April 2019). "New insights into the ORF2 capsid protein, a key player of the hepatitis E virus lifecycle". Scientific Reports. 9 (1): 6243. Bibcode:2019NatSR...9.6243A. doi:10.1038/s41598-019-42737-2. ISSN 2045-2322. PMC 6472401. PMID 31000788.
- ↑ Ahmad, Imran; Holla, R. Prasida; Jameel, Shahid (October 2011). "Molecular Virology of Hepatitis E Virus". Virus Research. 161 (1): 47–58. doi:10.1016/j.virusres.2011.02.011. ISSN 0168-1702. PMC 3130092. PMID 21345356.
- ↑ Ding, Qiang; Heller, Brigitte; Capuccino, Juan M. V.; Song, Bokai; Nimgaonkar, Ila; Hrebikova, Gabriela; Contreras, Jorge E.; Ploss, Alexander (2017). "Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles". Proceedings of the National Academy of Sciences of the United States of America. 114 (5): 1147–1152. doi:10.1073/pnas.1614955114. ISSN 1091-6490. PMC 5293053. PMID 28096411.
- 1 2 Cao, Dianjun; Meng, Xiang-Jin (2012-08-22). "Molecular biology and replication of hepatitis E virus". Emerging Microbes & Infections. 1 (8): e17. doi:10.1038/emi.2012.7. PMC 3630916. PMID 26038426.
- ↑ Tao, TS; Liu, Z; Ye, Q; Mata, DA; Li, K; Yin, C; Zhang, J; Tao, YJ (Aug 4, 2009). "Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding". Proceedings of the National Academy of Sciences of the United States of America. 106 (31): 12992–7. Bibcode:2009PNAS..10612992G. doi:10.1073/pnas.0904848106. PMC 2722310. PMID 19622744.
- 1 2 3 "Hepatitis E Questions and Answers for Health Professionals". www.cdc.gov. CDC. 13 June 2018. Archived from the original on 8 March 2016. Retrieved 9 September 2017.
- ↑ Aggarwal, Rakesh (2 October 2012). "Diagnosis of hepatitis E". Nature Reviews Gastroenterology & Hepatology. 10 (1): 24–33. doi:10.1038/nrgastro.2012.187. ISSN 1759-5045. PMID 23026902. S2CID 11958858. Archived from the original on 28 August 2021. Retrieved 31 January 2020.subscription needed
- ↑ Baylis, Sally A.; Blümel, Johannes; Mizusawa, Saeko; Matsubayashi, Keiji; Sakata, Hidekatsu; Okada, Yoshiaki; Nübling, C. Micha; Hanschmann, Kay-Martin O. (May 2013). "World Health Organization International Standard to Harmonize Assays for Detection of Hepatitis E Virus RNA". Emerging Infectious Diseases. 19 (5): 729–735. doi:10.3201/eid1905.121845. ISSN 1080-6040. PMC 3647515. PMID 23647659.
- ↑ Webb, Glynn W.; Dalton, Harry R. (3 April 2019). "Hepatitis E: an underestimated emerging threat". Therapeutic Advances in Infectious Disease. 6: 204993611983716. doi:10.1177/2049936119837162. ISSN 2049-9361. PMC 6448100. PMID 30984394.
- ↑ Hillyer, Christopher D.; Shaz, Beth H.; Zimring, James C.; Abshire, Thomas C. (2009). Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects. Elsevier. p. 364. ISBN 9780080922300. Archived from the original on 2020-08-03. Retrieved 2019-08-22.
- ↑ Dreier, Jens; Knabbe, Cornelius; Vollmer, Tanja (1 February 2018). "Transfusion-Transmitted Hepatitis E: NAT Screening of Blood Donations and Infectious Dose". Frontiers in Medicine. 5: 5. doi:10.3389/fmed.2018.00005. ISSN 2296-858X. PMC 5799287. PMID 29450199.
- ↑ Hans, Rekha; Marwaha, Neelam (2014). "Nucleic acid testing-benefits and constraints". Asian Journal of Transfusion Science. 8 (1): 2–3. doi:10.4103/0973-6247.126679. ISSN 0973-6247. PMC 3943139. PMID 24678164.
- ↑ Shrestha MP, Scott RM, Joshi DM (2007). "Safety and efficacy of a recombinant hepatitis E vaccine". New England Journal of Medicine. 356 (9): 895–903. doi:10.1056/NEJMoa061847. PMID 17329696.
- 1 2 Park, S. B. (November 2012). "Hepatitis E vaccine debuts". Nature. 491 (7422): 21–22. Bibcode:2012Natur.491...21P. doi:10.1038/491021a. PMID 23128204.
- ↑ Labrique, Alain B.; Sikder, Shegufta S.; Krain, Lisa J.; West, Keith P.; Christian, Parul; Rashid, Mahbubur; Nelson, Kenrad E. (2012-09-01). "Hepatitis E, a Vaccine-Preventable Cause of Maternal Deaths". Emerging Infectious Diseases. 18 (9): 1401–1404. doi:10.3201/eid1809.120241. ISSN 1080-6040. PMC 3437697. PMID 22931753.
- 1 2 "Hepatitis E vaccine: WHO position paper, May 2015" (PDF). Relevé Épidémiologique Hebdomadaire. 90 (18): 185–200. 1 May 2015. PMID 25935931. Archived (PDF) from the original on 14 August 2020. Retrieved 5 October 2020.
- ↑ Dalton, Harry R.; Kamar, Nassim (2016). "Treatment of hepatitis E virus". Current Opinion in Infectious Diseases. 29 (6): 639–644. doi:10.1097/QCO.0000000000000316. ISSN 1473-6527. PMID 27607911. S2CID 25304902.
- ↑ Peters van Ton, A. M.; Gevers, T. J. G.; Drenth, J. P. H. (Dec 2015). "Antiviral therapy in chronic hepatitis E: a systematic review". Journal of Viral Hepatitis. 22 (12): 965–973. doi:10.1111/jvh.12403. ISSN 1365-2893. PMID 25760481. S2CID 10199788.
- ↑ Teshale, Eyasu H. (31 May 2017). "Hepatitis E – Infectious Diseases Related to Travel Chapter 3 – 2018 Yellow Book". www.cdc.gov. CDC. Archived from the original on 15 June 2019. Retrieved 27 July 2019.
- ↑ Navaneethan, Udayakumar; Mohajer, Mayar Al; Shata, Mohamed T (2008). "Hepatitis E and Pregnancy- Understanding the pathogenesis". Liver International. 28 (9): 1190–1199. doi:10.1111/j.1478-3231.2008.01840.x. ISSN 1478-3223. PMC 2575020. PMID 18662274.
- ↑ Public Health England, infection report. "Common animal associated infections quarterly report (England and Wales) – fourth quarter 2015" (PDF). gov.uk. Archived (PDF) from the original on 27 July 2019. Retrieved 27 July 2019.
- 1 2 Teshale EH, Howard CM, Grytdal SP (2010). "Hepatitis E epidemic, Uganda". Emerging Infect. Dis. 16 (1): 126–129. doi:10.3201/eid1601.090764. PMC 2874362. PMID 20031058.
- ↑ Investigation of Hepatitis E Outbreak Among Refugees — Upper Nile, South Sudan, 2012–2013. www.cdc.gov (Report). Vol. 62. CDC Morbidity and Mortality Weekly Report. 26 July 2013. pp. 581–586. Archived from the original on 24 June 2017. Retrieved 9 September 2017.
- ↑ Hereward Holland (2 February 2013). "Hepatitis outbreak kills 88 in South Sudan—aid agency". Reuters. Archived from the original on 28 December 2013. Retrieved 6 February 2013.
- ↑ Sharma, Christopher (5 September 2014). "Nepal, hepatitis E epidemic: 9 dead and over 6 thousand infected". www.asianews.it. Archived from the original on 28 May 2019. Retrieved 27 July 2019.
- ↑ "Hepatitis E cases in Namibia rise to 490". www.xinhuanet.com. Xinhua News. 28 January 2018. Archived from the original on 29 January 2018. Retrieved 28 January 2018.
- ↑ "Weekly bulletins on outbreaks and other emergencies" (PDF). World Health Organization. WHO.int. Archived (PDF) from the original on 7 March 2020. Retrieved 20 May 2019.
- ↑ "Outbreaks and Emergencies Bulletin, Week 33: 12 – 18 August 2019". WHO | Regional Office for Africa. Archived from the original on 20 August 2019. Retrieved 20 August 2019.
- ↑ CNN, Jessie Yeung. "Rats are infecting humans with hepatitis, and nobody knows how". CNN. Archived from the original on 9 May 2020. Retrieved 11 May 2020.
- ↑ Khudyakov, Yury E.; Purdy, Michael A. (2010). "Evolutionary History and Population Dynamics of Hepatitis E Virus". PLOS ONE. 5 (12): e14376. Bibcode:2010PLoSO...514376P. doi:10.1371/journal.pone.0014376. ISSN 1932-6203. PMC 3006657. PMID 21203540.
- ↑ Baha, Sarra; Behloul, Nouredine; Liu, Zhenzhen; Wei, Wenjuan; Shi, Ruihua; Meng, Jihong (2019-10-29). "Comprehensive analysis of genetic and evolutionary features of the hepatitis E virus". BMC Genomics. 20 (1): 790. doi:10.1186/s12864-019-6100-8. ISSN 1471-2164. PMC 6820953. PMID 31664890.
- ↑ Mirazo S, Mir D, Bello G, Ramos N, Musto H, Arbiza J (2016). "New insights into the hepatitis E virus genotype 3 phylodynamics and evolutionary history". Infect Genet Evol. 43: 267–273. doi:10.1016/j.meegid.2016.06.003. PMID 27264728.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article incorporates public domain text from the CDC as cited
Further reading
- Parvez, Mohammad Khalid (2013-01-01). "Chronic hepatitis E infection: risks and controls". Intervirology. 56 (4): 213–216. doi:10.1159/000349888. ISSN 1423-0100. PMID 23689166. Archived from the original on 2017-09-22. Retrieved 2017-09-21.
- Aggarwa, Rakesh; Gandhi, Sanjay (2010). "A systematic review on prevalence of hepatitis E disease and seroprevalence of hepatitis E virus antibody" (PDF). World Health Organization. WHO/IVB/10.14. Archived (PDF) from the original on 2013-02-19. Retrieved 2012-02-12.
External links
| Classification | |
|---|---|
| External resources |