Phosphorus trichloride
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Phosphorus trichloride | |
| Systematic IUPAC name
Trichlorophosphane | |
| Other names
Phosphorus(III) chloride Phosphorous chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.864 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1809 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PCl3 | |
| Molar mass | 137.33 g/mol |
| Appearance | Colorless to yellow fuming liquid[1] |
| Odor | unpleasant, acrid, like hydrochloric acid[1] |
| Density | 1.574 g/cm3 |
| Melting point | −93.6 °C (−136.5 °F; 179.6 K) |
| Boiling point | 76.1 °C (169.0 °F; 349.2 K) |
| hydrolyzes | |
| Solubility in other solvents | soluble in benzene, CS2, ether, chloroform, CCl4, halogenated organic solvents reacts with ethanol |
| Vapor pressure | 13.3 kPa |
| −63.4·10−6 cm3/mol | |
Refractive index (nD) |
1.5122 (21 °C) |
| Viscosity | 0.65 cP (0 °C) 0.438 cP (50 °C) |
| 0.97 D | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−319.7 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Highly toxic,[2] corrosive |
| GHS labelling:[3] | |
   | |
| Danger | |
| H300, H301, H314, H330, H373 | |
| P260, P273, P284, P303+P361+P353, P304+P340+P310, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
18 mg/kg (rat, oral)[4] |
LC50 (median concentration) |
104 ppm (rat, 4 hr) 50 ppm (guinea pig, 4 hr)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.5 ppm (3 mg/m3)[1] |
REL (Recommended) |
TWA 0.2 ppm (1.5 mg/m3) ST 0.5 ppm (3 mg/m3)[1] |
IDLH (Immediate danger) |
25 ppm[1] |
| Safety data sheet (SDS) | ICSC 0696 |
| Related compounds | |
Related phosphorus chlorides |
Phosphorus pentachloride Phosphorus oxychloride Diphosphorus tetrachloride |
Related compounds |
Phosphorus trifluoride Phosphorus tribromide Phosphorus triiodide |
| Supplementary data page | |
| Phosphorus trichloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.
History
Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus.[5] Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas.[6]
Preparation
World production exceeds one-third of a million tonnes.[7] Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5.
- P4 + 6 Cl2 → 4 PCl3
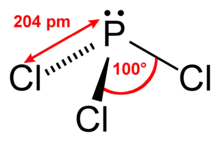
Structure and spectroscopy
It has a trigonal pyramidal shape. Its 31P NMR spectrum exhibits a singlet around +220 ppm with reference to a phosphoric acid standard.
Reactions
The phosphorus in PCl3 is often considered to have the +3 oxidation state and the chlorine atoms are considered to be in the −1 oxidation state. Most of its reactivity is consistent with this description.[8]
Oxidation
PCl3 is a precursor to other phosphorus compounds, undergoing oxidation to phosphorus pentachloride (PCl5), thiophosphoryl chloride (PSCl3), or phosphorus oxychloride (POCl3).
PCl3 as an electrophile
PCl3 reacts vigorously with water to form phosphorous acid (H3PO3) and hydrochloric acid:
- PCl3 + 3 H2O → H3PO3 + 3 HCl
Phosphorus trichloride is the precursor to organophosphorus compounds. It reacts with phenol to give triphenyl phosphite:
- 3 PhOH + PCl3 → P(OPh)3 + 3 HCl (Ph = C6H5)
Alcohols such as ethanol react similarly in the presence of a base such as a tertiary amine:[9]
- PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH+Cl−
With one equivalent of alcohol and in the absence of base, the first product is alkoxyphosphorodichloridite:[10]
- PCl3 + EtOH → PCl2(OEt) + HCl
In the absence of base, however, with excess alcohol, phosphorus trichloride converts to diethylphosphite:[11][12]
- PCl3 + 3 EtOH → (EtO)2P(O)H + 2 HCl + EtCl
Secondary amines (R2NH) form aminophosphines. For example, bis(diethylamino)chlorophosphine, (Et2N)2PCl, is obtained from diethylamine and PCl3. Thiols (RSH) form P(SR)3. An industrially relevant reaction of PCl3 with amines is phosphonomethylation, which employs formaldehyde:
- R2NH + PCl3 + CH2O → (HO)2P(O)CH2NR2 + 3 HCl
The herbicide glyphosate is also produced this way.
The reaction of PCl3 with Grignard reagents and organolithium reagents is a useful method for the preparation of organic phosphines with the formula R3P (sometimes called phosphanes) such as triphenylphosphine, Ph3P.
- 3 RMgBr + PCl3 → R3P + 3 MgBrCl
Triphenylphosphine is produced industrially by the reaction between phosphorus trichlorid, chlorobenzene, and sodium:[13]
- PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl, where Ph = C6H5
Under controlled conditions or especially with bulky R groups, similar reactions afford less substituted derivatives such as chlorodiisopropylphosphine.
Conversion of alcohols to alkyl chlorides
Phosphorus trichloride is commonly used to convert primary and secondary alcohols to the corresponding chlorides.[14] As discussed above, the reaction of alcohols with phosphorus trichloride is sensitive to conditions. The mechanism for the ROH →RCl conversion involves the reaction of HCl with phosphite esters:
- P(OR)3 + HCl ⇌ HP(OR)+3Cl−
- HP(OR)+3Cl− → RCl + HOP(OR)2.
- HOP(OR)2 + HCl ⇌ H2OP(OR)+2Cl−
- H2OP(OR)+2Cl− → RCl + (HO)2P(OR)
- (HO)2P(OR) + HCl ⇌ H(HO)2P(OR)+Cl−
- H(HO)2P(OR)+Cl− → RCl + (HO)3P
The first step proceeds with nearly ideal stereochemistry but the final step far less so owing to an SN2 pathway.
Redox reactions
Phosphorus trichloride undergoes a variety of redox reactions:[13]
- 3PCl3 + 2 CrO3 → 3POCl3 + Cr2O3
- PCl3 + SO3 → POCl3 + SO2
- 3 PCl3 + SO2 → 2POCl3 + PSCl3
PCl3 as a nucleophile
Phosphorus trichloride has a lone pair, and therefore can act as a Lewis base,[15] e.g., forming a 1:1 adduct Br3B-PCl3. Metal complexes such as Ni(PCl3)4 are known, again demonstrating the ligand properties of PCl3.
This Lewis basicity is exploited in the Kinnear–Perren reaction to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and alkylphosphonate esters (RP(O)(OR')2). Alkylation of phosphorus trichloride is effected in the presence of aluminium trichloride give the alkyltrichlorophosphonium salts, which are versatile intermediates:[16]
- PCl3 + RCl + AlCl3 → RPCl+
3 + AlCl−
4
The RPCl+
3 product can then be decomposed with water to produce an alkylphosphonic dichloride RP(=O)Cl2.
PCl3 as a ligand
PCl3, like the more popular phosphorus trifluoride, is a ligand in coordination chemistry. One example is Mo(CO)5PCl3.[17]
Uses
PCl3 is important indirectly as a precursor to PCl5, POCl3 and PSCl3, which are used in many applications, including herbicides, insecticides, plasticisers, oil additives, and flame retardants.
For example, oxidation of PCl3 gives POCl3, which is used for the manufacture of triphenyl phosphate and tricresyl phosphate, which find application as flame retardants and plasticisers for PVC. They are also used to make insecticides such as diazinon. Phosphonates include the herbicide glyphosate.
PCl3 is the precursor to triphenylphosphine for the Wittig reaction, and phosphite esters which may be used as industrial intermediates, or used in the Horner-Wadsworth-Emmons reaction, both important methods for making alkenes. It can be used to make trioctylphosphine oxide (TOPO), used as an extraction agent, although TOPO is usually made via the corresponding phosphine.
PCl3 is also used directly as a reagent in organic synthesis. It is used to convert primary and secondary alcohols into alkyl chlorides, or carboxylic acids into acyl chlorides, although thionyl chloride generally gives better yields than PCl3.[18]
Safety
- 600 ppm is lethal in just a few minutes.[19]
- 25 ppm is the US NIOSH "Immediately Dangerous to Life and Health" level[20]
- 0.5 ppm is the US OSHA "permissible exposure limit" over a time-weighted average of 8 hours.[21]
- 0.2 ppm is the US NIOSH "recommended exposure limit" over a time-weighted average of 8 hours.[22]
- Under EU Directive 67/548/EEC, PCl3 is classified as very toxic and corrosive , and the risk phrases R14, R26/28, R35 and R48/20 are obligatory.
Industrial production of phosphorus trichloride is controlled under the Chemical Weapons Convention, where it is listed in schedule 3, as it can be used to produce mustard agents.[23]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0511". National Institute for Occupational Safety and Health (NIOSH).
- Phosphorus trichloride toxicity
- Sigma-Aldrich Co., Phosphorus trichloride.
- "Phosphorus trichloride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Gay-Lussac; Thénard (27 May 1808). "Extrait de plusieurs notes sur les métaux de la potasse et de la soude, lues à l'Institut depuis le 12 janvier jusqu'au 16 mai" [Extracts from several notes on the metals potassium and sodium, read at the Institute from the 12th of January to the 16th of May]. Gazette Nationale, Ou le Moniteur Universel (in French). 40 (148): 581–582. From p. 582: "Seulement ils ont rapporté qu'en traitant le mercure doux par le phosphure, dans l'espérance d'avoir de l'acide muriatique bien sec, il ont trouvé une liqueur nouvelle très limpide, sans couleur, répandant de fortes vapeurs, s'enflammant spontanément lorsqu'on en imbibe le papier joseph; laquelle ne paraît être qu'une combinaison de phosphore, d'oxigène et d'acide muriatique, et par conséquent analogue à cette qu'on obtient en traitant le soufre par le gas acide muriatique oxigèné." (Only they reported that by treating calomel with phosphorus, in the hope of obtaining very dry hydrogen chloride, they found a new, very clear liquid, colorless, giving off strong vapors, spontaneously igniting when one soaks filter paper in it; which seems to be only a compound of phosphorus, oxygen, and hydrochloric acid, and thus analogous to what one obtains by treating sulfur with chlorine gas.)
- Davy, Humphry (1809). "The Bakerian Lecture. An account of some new analytical researches on the nature of certain bodies, particularly the alkalies, phosphorus, sulphur, carbonaceous matter, and the acids hitherto undecomposed; with some general observations on chemical theory". Philosophical Transactions of the Royal Society of London. 99: 39–104. doi:10.1098/rstl.1809.0005. S2CID 98814859. On pp. 94–95, Davy mentioned that when he burned phosphorus in chlorine gas ("oxymuriatic acid gas"), he obtained a clear liquid (phosphorus trichloride) and a white solid (phosphorus pentachloride).
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- "Phosphorus trichloride (PCl3)". www.simply.science. Retrieved 15 February 2023.
- A. H. Ford-Moore & B. J. Perry (1963). "Triethyl Phosphite". Organic Syntheses.; Collective Volume, vol. 4, p. 955
- Gerrard, W.; Isaacs, M. J. D.; Machell, G.; Smith, K. B.; Wyvill, P. L. (1953). "394. Interaction of phosphorus trichloride with alcohols and with hydroxy-esters". Journal of the Chemical Society (Resumed): 1920. doi:10.1039/JR9530001920.
- Malowan, John E. (1953). "Diethyl phosphite". Inorganic Syntheses. Vol. 4. pp. 58–60. doi:10.1002/9780470132357.ch19. ISBN 9780470132357.
- Pedrosa, Leandro (2011). "Esterification of Phosphorus Trichloride with Alcohols; Diisopropyl phosphonate". ChemSpider Synthetic Pages. Royal Society of Chemistry: 488. doi:10.1039/SP488.
- Corbridge, D. E. C. (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- Brown, Geoffrey W. (1971). "Displacement of Hydroxyl Groups". The Hydroxyl Group (1971). PATai's Chemistry of Functional Groups. pp. 593–639. doi:10.1002/9780470771259.ch11. ISBN 9780470771259.
- R. R. Holmes (1960). "An examination of the basic nature of the trihalides of phosphorus, arsenic and antimony". Journal of Inorganic and Nuclear Chemistry. 12 (3–4): 266–275. doi:10.1016/0022-1902(60)80372-7.
- Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_545.pub2.
- Frenking, Gernot; Wichmann, Karin; Fröhlich, Nikolaus; Grobe, Joseph; Golla, Winfried; Van, Duc Le; Krebs, Bernt; Läge, Mechtild (2002). "Nature of the Metal−Ligand Bond in M(CO)5PX3 Complexes (M = Cr, Mo, W; X = H, Me, F, Cl): Synthesis, Molecular Structure, and Quantum-Chemical Calculations". Organometallics. 21 (14): 2921–2930. doi:10.1021/om020311d.
- L. G. Wade Jr. (2005). Organic Chemistry (6th ed.). Upper Saddle River, New Jersey, USA: Pearson/Prentice Hall. p. 477.
- A. D. F. Toy (1973). The Chemistry of Phosphorus. Oxford, UK: Pergamon Press.
- Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
- OSHA: Phosphorus Trichloride
- CDC - NIOSH Pocket Guide to Chemical Hazards
- M. C. Forbes; C. A. Roswell; R. N. Maxson (2007). "Phosphorus(III) Chloride". Inorganic Syntheses. pp. 145–7. doi:10.1002/9780470132333.ch42. ISBN 9780470132333.
{{cite book}}:|journal=ignored (help)
