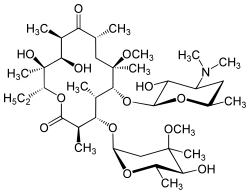
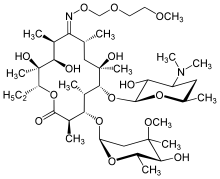
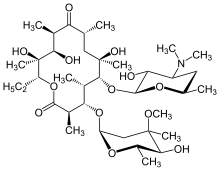
Macrolide
The macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic.[1]
Macrolides are bacteriostatic in that they suppress or inhibit bacterial growth rather than killing bacteria completely.
Definition
In general, any macrocyclic lactone having greater than 8-membered rings are candidates for this class. The macrocycle may contain amino nitrogen, amide nitrogen (but should be differentiated from cyclopeptides), an oxazole ring, or a thiazole ring. Benzene rings are excluded, in order to differentiate from tannins. Also lactams instead of lactones (as in the ansamycin family) are excluded. Included are not only 12-16 membered macrocycles but also larger rings as in tacrolimus.[2]
History
The first macrolide discovered was erythromycin, which was first used in 1952. Erythromycin was widely used as a substitute to penicillin in cases where patients were allergic to penicillin or had penicillin-resistant illnesses. Later macrolides developed, including azithromycin and clarithromycin, stemmed from chemically modifying erythromycin; these compounds were designed to be more easily absorbed and have fewer side-effects (erythromycin caused gastrointestinal side-effects in a significant proportion of users). [3]
Uses
Antibiotic macrolides are used to treat infections caused by Gram-positive bacteria (e.g., Streptococcus pneumoniae) and limited Gram-negative bacteria (e.g., Bordetella pertussis, Haemophilus influenzae), and some respiratory tract and soft-tissue infections.[4] The antimicrobial spectrum of macrolides is slightly wider than that of penicillin, and, therefore, macrolides are a common substitute for patients with a penicillin allergy. Beta-hemolytic streptococci, pneumococci, staphylococci, and enterococci are usually susceptible to macrolides. Unlike penicillin, macrolides have been shown to be effective against Legionella pneumophila, mycoplasma, mycobacteria, some rickettsia, and chlamydia.
Macrolides are not to be used on nonruminant herbivores, such as horses and rabbits. They rapidly produce a reaction causing fatal digestive disturbance.[5] It can be used in horses less than one year old, but care must be taken that other horses (such as a foal's mare) do not come in contact with the macrolide treatment.
Macrolides can be administered in a variety of ways, including tablets, capsules, suspensions, injections and topically.[6]
Mechanism of action
Antibacterial
Macrolides are protein synthesis inhibitors. The mechanism of action of macrolides is inhibition of bacterial protein biosynthesis, and they are thought to do this by preventing peptidyltransferase from adding the growing peptide attached to tRNA to the next amino acid[7] (similarly to chloramphenicol[8]) as well as inhibiting bacterial ribosomal translation.[7] Another potential mechanism is premature dissociation of the peptidyl-tRNA from the ribosome.[9]
Macrolide antibiotics do so by binding reversibly to the P site on the 50S subunit of the bacterial ribosome. This action is considered to be bacteriostatic. Macrolides are actively concentrated within leukocytes, and thus are transported into the site of infection.[10]
Diffuse panbronchiolitis
The macrolide antibiotics erythromycin, clarithromycin, and roxithromycin have proven to be an effective long-term treatment for the idiopathic, Asian-prevalent lung disease diffuse panbronchiolitis (DPB).[11][12] The successful results of macrolides in DPB stems from controlling symptoms through immunomodulation (adjusting the immune response),[12] with the added benefit of low-dose requirements.[11]
With macrolide therapy in DPB, great reduction in bronchiolar inflammation and damage is achieved through suppression of not only neutrophil granulocyte proliferation but also lymphocyte activity and obstructive secretions in airways.[11] The antimicrobial and antibiotic effects of macrolides, however, are not believed to be involved in their beneficial effects toward treating DPB.[13] This is evident, as the treatment dosage is much too low to fight infection, and in DPB cases with the occurrence of the macrolide-resistant bacterium Pseudomonas aeruginosa, macrolide therapy still produces substantial anti-inflammatory results.[11]
Examples
Antibiotic macrolides
US FDA-approved :
- Azithromycin - unique; does not extensively inhibit CYP3A4
- Clarithromycin
- Erythromycin
Non-US FDA-approved:
- Carbomycin A
- Josamycin
- Kitasamycin
- Midecamycin/midecamycin acetate
- Oleandomycin
- Solithromycin
- Spiramycin - approved in the EU, and in other countries
- Troleandomycin - used in Italy and Turkey
- Tylosin/tylocine - used in animals
- Roxithromycin
Ketolides
Ketolides are a class of antibiotics that are structurally related to the macrolides. They are used to treat respiratory tract infections caused by macrolide-resistant bacteria. Ketolides are especially effective, as they have two ribosomal binding sites.
Ketolides include:
- Telithromycin - the first and only approved ketolide[14]
- Cethromycin
- Solithromycin
Fluoroketolides
Fluoroketolides are a class of antibiotics that are structurally related to the ketolides. The fluoroketolides have three ribosomal interaction sites.
Fluoroketolides include:
- Solithromycin - the first and currently the only fluoroketolide (not yet approved)
Non-antibiotic macrolides
The drugs tacrolimus, pimecrolimus, and sirolimus, which are used as immunosuppressants or immunomodulators, are also macrolides. They have similar activity to ciclosporin.
Antifungal drugs
Polyene antimycotics, such as amphotericin B, nystatin etc., are a subgroup of macrolides.[15] Cruentaren is another example of an antifungal macrolide.[16]
Toxic macrolides
A variety of toxic macrolides produced by bacteria have been isolated and characterized, such as the mycolactones.
Resistance
The primary means of bacterial resistance to macrolides occurs by post-transcriptional methylation of the 23S bacterial ribosomal RNA. This acquired resistance can be either plasmid-mediated or chromosomal, i.e., through mutation, and results in cross-resistance to macrolides, lincosamides, and streptogramins (an MLS-resistant phenotype).[17]
Two other types of acquired resistance rarely seen include the production of drug-inactivating enzymes (esterases or kinases), as well as the production of active ATP-dependent efflux proteins that transport the drug outside of the cell.
Azithromycin has been used to treat strep throat (Group A streptococcal (GAS) infection caused by Streptococcus pyogenes) in penicillin-sensitive patients, however macrolide-resistant strains of GAS are not uncommon. Cephalosporin is another option for these patients.
Side-effects
A 2008 British Medical Journal article highlights that the combination of some macrolides and statins (used for lowering cholesterol) is not advisable and can lead to debilitating myopathy.[18] This is because some macrolides (clarithromycin and erythromycin, not azithromycin) are potent inhibitors of the cytochrome P450 system, particularly of CYP3A4. Macrolides, mainly erythromycin and clarithromycin, also have a class effect of QT prolongation, which can lead to torsades de pointes. Macrolides exhibit enterohepatic recycling; that is, the drug is absorbed in the gut and sent to the liver, only to be excreted into the duodenum in bile from the liver. This can lead to a buildup of the product in the system, thereby causing nausea. In infants the use of erythromycin has been associated with pyloric stenosis.[19][20]
Some macrolides are also known to cause cholestasis, a condition where bile cannot flow from the liver to the duodenum.[21] A new study found an association between erythromycin use during infancy and developing IHPS (Infantile hypertrophic pyloric stenosis) in infants.[22] However, no significant association was found between macrolides use during pregnancy or breastfeeding.[22]
A Cochrane review showed gastrointestinal symptoms to be the most frequent adverse event reported in literature.[23]
Interactions
Macrolides should not be taken with colchicine as it may lead to colchicine toxicity. Symptoms of colchicine toxicity include gastrointestinal upset, fever, myalgia, pancytopenia, and organ failure.[24]
References
- Arriola Apelo SI, Lamming DW (July 2016). "apamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island". J Gerontol A Biol Sci Med Sci. 71 (7): 841–9. doi:10.1093/gerona/glw090. PMC 4906330. PMID 27208895. Retrieved 17 July 2022.
- Omura S, ed. (2002). Macrolide Antibiotics: Chemistry, Biology, and Practice (2nd ed.). Academic Press. ISBN 978-0-1252-6451-8.
- Klein JO (April 1997). "History of macrolide use in pediatrics". The Pediatric Infectious Disease Journal. 16 (4): 427–31. doi:10.1097/00006454-199704000-00025. PMID 9109154.
- "Macrolide Antibiotics Comparison: Erythromycin, Clarithromycin, Azithromycin". Retrieved 22 March 2017.
- Giguere S, Prescott JF, Baggot JD, Walker RD, Dowling PM, eds. (2006). Antimicrobial Therapy in Veterinary Medicine (4th ed.). Wiley-Blackwell. ISBN 978-0-8138-0656-3.
- "DailyMed". Food and Drug Administration (US). Retrieved 22 March 2017.
- Protein synthesis inhibitors: macrolides mechanism of action animation. Classification of agents Pharmamotion. Author: Gary Kaiser. The Community College of Baltimore County. Retrieved on July 31, 2009
- Drainas D, Kalpaxis DL, Coutsogeorgopoulos C (April 1987). "Inhibition of ribosomal peptidyltransferase by chloramphenicol. Kinetic studies". European Journal of Biochemistry. 164 (1): 53–8. doi:10.1111/j.1432-1033.1987.tb10991.x. PMID 3549307.
- Tenson T, Lovmar M, Ehrenberg M (July 2003). "The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome". Journal of Molecular Biology. 330 (5): 1005–14. doi:10.1016/S0022-2836(03)00662-4. PMID 12860123.
- Bailly S, Pocidalo JJ, Fay M, Gougerot-Pocidalo MA (October 1991). "Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin". Antimicrobial Agents and Chemotherapy. 35 (10): 2016–9. doi:10.1128/AAC.35.10.2016. PMC 245317. PMID 1759822.
- Keicho N, Kudoh S (2002). "Diffuse panbronchiolitis: role of macrolides in therapy". American Journal of Respiratory Medicine. 1 (2): 119–31. doi:10.1007/BF03256601. PMID 14720066.
- López-Boado YS, Rubin BK (June 2008). "Macrolides as immunomodulatory medications for the therapy of chronic lung diseases". Current Opinion in Pharmacology. 8 (3): 286–91. doi:10.1016/j.coph.2008.01.010. PMID 18339582.
- Schultz MJ (July 2004). "Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis". The Journal of Antimicrobial Chemotherapy. 54 (1): 21–8. doi:10.1093/jac/dkh309. PMID 15190022.
- Nguyen M, Chung EP (August 2005). "Telithromycin: the first ketolide antimicrobial". Clinical Therapeutics. 27 (8): 1144–63. doi:10.1016/j.clinthera.2005.08.009. PMID 16199242.
- Hamilton-Miller JM (June 1973). "Chemistry and biology of the polyene macrolide antibiotics". Bacteriological Reviews. 37 (2): 166–96. doi:10.1128/br.37.3.166-196.1973. PMC 413810. PMID 4578757.
- Kunze B, Sasse F, Wieczorek H, Huss M (July 2007). "Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases". FEBS Letters. 581 (18): 3523–7. doi:10.1016/j.febslet.2007.06.069. PMID 17624334.
- Munita JM, Arias CA (April 2016). "Mechanisms of Antibiotic Resistance". Microbiology Spectrum. 4 (2): 481–511. doi:10.1128/microbiolspec.VMBF-0016-2015. ISBN 9781555819279. PMC 4888801. PMID 27227291.
- Sathasivam S, Lecky B (November 2008). "Statin induced myopathy". BMJ. 337: a2286. doi:10.1136/bmj.a2286. PMID 18988647. S2CID 3239804.
- SanFilippo A (April 1976). "Infantile hypertrophic pyloric stenosis related to ingestion of erythromycine estolate: A report of five cases". Journal of Pediatric Surgery. 11 (2): 177–80. doi:10.1016/0022-3468(76)90283-9. PMID 1263054.
- Honein MA, Paulozzi LJ, Himelright IM, Lee B, Cragan JD, Patterson L, Correa A, Hall S, Erickson JD (1999). "Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study". Lancet. 354 (9196): 2101–5. doi:10.1016/S0140-6736(99)10073-4. PMID 10609814. S2CID 24160212.
- Hautekeete ML (1995). "Hepatotoxicity of antibiotics". Acta Gastro-Enterologica Belgica. 58 (3–4): 290–6. PMID 7491842.
- Abdellatif M, Ghozy S, Kamel MG, Elawady SS, Ghorab MM, Attia AW, Le Huyen TT, Duy DT, Hirayama K, Huy NT (March 2019). "Association between exposure to macrolides and the development of infantile hypertrophic pyloric stenosis: a systematic review and meta-analysis". European Journal of Pediatrics. 178 (3): 301–314. doi:10.1007/s00431-018-3287-7. PMID 30470884. S2CID 53711818.
- Hansen, Malene Plejdrup; Scott, Anna M; McCullough, Amanda; Thorning, Sarah; Aronson, Jeffrey K; Beller, Elaine M; Glasziou, Paul P; Hoffmann, Tammy C; Clark, Justin; Del Mar, Chris B (18 January 2019). "Adverse events in people taking macrolide antibiotics versus placebo for any indication". Cochrane Database of Systematic Reviews. 1: CD011825. doi:10.1002/14651858.CD011825.pub2. PMC 6353052. PMID 30656650.
- John R. Horn & Philip D. Hansten (2006). "Life Threatening Colchicine Drug Interactions. Drug Interactions: Insights and Observations" (PDF).
Further reading
- Ōmura S (2002). Macrolide antibiotics: chemistry, biology, and practice (2nd ed.). Boston: Academic Press. ISBN 978-0-12-526451-8.
- Bryskier A. "Antibacterial Agents; Structure Activity Relationships" (PDF). p. 143. Archived from the original (PDF) on 2006-03-04.