Cardiac fibrosis
Cardiac fibrosis commonly refers to the excess deposition of extracellular matrix in the cardiac muscle, but the term may also refer to an abnormal thickening of the heart valves due to inappropriate proliferation of cardiac fibroblasts.[1] Fibrotic cardiac muscle is stiffer and less compliant and is seen in the progression to heart failure. The description below focuses on a specific mechanism of valvular pathology but there are other causes of valve pathology and fibrosis of the cardiac muscle.
Fibrocyte cells normally secrete collagen, and function to provide structural support for the heart. When over-activated this process causes thickening and fibrosis of the valve, with white tissue building up primarily on the tricuspid valve, but also occurring on the pulmonary valve. The thickening and loss of flexibility eventually may lead to valvular dysfunction and right-sided heart failure.
Types
Following are types of myocardial fibrosis:
- Interstitial fibrosis, which is unspecific, and has been described in congestive heart failure, hypertension, and normal aging.[2]
- Subepicardial fibrosis, also unspecific, and is associated with non-infarction diagnoses such as myocarditis and non-ischemic cardiomyopathy.[3][4]
- Replacement fibrosis, which indicates an older infarction.[2]
 Micrograph of healthy myocardium versus interstitial fibrosis in dilated cardiomyopathy. Alcian blue stain. The fibrosis is either evenly distributed between myocytes or follows anatomic structures such as blood vessels.
Micrograph of healthy myocardium versus interstitial fibrosis in dilated cardiomyopathy. Alcian blue stain. The fibrosis is either evenly distributed between myocytes or follows anatomic structures such as blood vessels.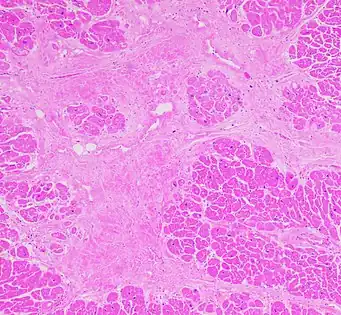 Interstitial fibrosis of chronic ischemic heart disease, H&E stain, with associated relatively well organized myocardial bundles
Interstitial fibrosis of chronic ischemic heart disease, H&E stain, with associated relatively well organized myocardial bundles Subepicardial fibrosis (epicardium at top).
Subepicardial fibrosis (epicardium at top). Replacement fibrosis in myocardial infarction, typically being boundless, dense and not conforming to the other types.
Replacement fibrosis in myocardial infarction, typically being boundless, dense and not conforming to the other types.
Connection with excess blood serotonin (5-HT)
Certain diseases such as neuroendocrine tumor of the small intestine (also known by the obsolete term carcinoid), which sometimes release large amounts of 5-hydroxytryptamine, commonly known as 5-HT or serotonin into the blood, may produce a characteristic pattern of mostly right-sided cardiac fibrosis which can be identified with echocardiography. Cardiac fibrosis is a significant source of morbidity and mortality in patients with functional neuroendocrine tumors. This pathology has also been seen in certain East-African tribes who eat foods (Matoke —a green banana) containing excess amounts of serotonin.
Connection with direct serotonergic agonist drugs
Elevated prevalence of cardiac fibrosis and related valvopathies was found to be associated with use of a number of unrelated drugs following long-term statistical analysis once the drugs had been on the market for some time. The cause of this was unknown at the time, but eventually it was realised that all the implicated drugs acted as agonists at 5-HT2B receptors in the heart in addition to their intended sites of action elsewhere in the body.[5][6]
The precise mechanisms involved remain elusive however, as while the cardiotoxicity shows some dose–response relationship,[7] it does not always develop, and consistent daily use over an extended period tends to be most strongly predictive of development of valvopathy.[8][9][10]
The drugs most classically associated with the condition are weight loss drugs such as fenfluramine and chlorphentermine, and antiparkinson drugs such as pergolide and cabergoline, which are prescribed for chronic use.
The heart valve changes seen with moderate and intermittent use can result in permanent damage and life-threatening heart problems if use of the causative drug is increased or continued, however longitudinal studies of former patients suggest that the damage will heal over time to some extent at least.[11][12]
Anorectics
Some appetite suppressant drugs such as fenfluramine (which in combination with phentermine was marketed as Pondimin and commonly referred to as fen-phen), chlorphentermine, and aminorex (along with its analogue 4-Methylaminorex which has seen sporadic use as a recreational drug) induce a similar pattern of cardiac fibrosis (and pulmonary hypertension), apparently by over-stimulating 5HT2B receptors on the cardiac fibroblast cells.
These drugs consequently tend to cause increased risk of heart valve damage and subsequent heart failure, which eventually led to them being withdrawn from the market.
Antimigraine drugs
Certain antimigraine drugs which are targeted at serotonin receptors as vasoconstrictive agents, have long been known to be associated with pulmonary hypertension and Raynaud's phenomenon (both vasoconstrictive effects), as well as retroperitoneal fibrosis (a fibrotic cell/fibrocyte proliferation effect, thought to be similar to cardiac valve fibrosis).
These drugs include ergotamine and methysergide and both drugs can also cause cardiac fibrosis.[13]
Antiparkinson drugs
Certain antiparkinson drugs, although targeted at dopaminergic receptors, cross-react with serotoninergic 5-HT2B receptors as well, and have been reported to cause cardiac fibrosis. These drugs include pergolide and cabergoline.
Antihypertensive drugs
Guanfacine may be a 5-HT2B agonist, based on the results of theoretical modeling and high-throughput screening.[14][15]
Pergolide
Pergolide was an antiparkinson medications that was in decreasing use since reported in 2003 to be associated with cardiac fibrosis.[16] In March 2007, pergolide was withdrawn from the U.S. market due to serious valvular damage that was shown in two independent studies.[17] [18]
Cabergoline
Like pergolide, cabergoline has been linked to cardiac damage. Among similar antiparkinsonian drugs, cabergoline exhibits the same type of serotonin receptor binding as pergolide.[19] Although lisuride, a related drug, also binds to the 5-HT2B receptor, it acts as an antagonist rather than as an agonist.[20]
In January 2007, cabergoline (Dostinex) was reported also to be associated with valvular proliferation heart damage.[21]
Recreational drugs
Several serotonergic recreational drugs, including the empathogens MDA and MDMA ("ecstasy"),[22] and some hallucinogens such as DOI[23] and Bromo-DragonFLY,[24] have all been shown to act as 5-HT2B agonists in vitro, but how significant this may be as a risk factor associated with their recreational use is unclear.
The piperazine derivative mCPP (a major metabolite of trazodone) is a 5-HT2B agonist in animal models, but actually behaves as a 5-HT2B antagonist in humans.[25][26][27]
MDMA
One study of human users of MDMA ("ecstasy") found that they did have heart valve changes suggestive of early cardiac fibrosis, which were not present in non-MDMA using controls,[28] suggesting that MDMA use certainly has the potential to cause this kind of heart damage.
On the other hand, there is as yet no statistical evidence to establish or negate significant increases in rates of cardiac valvopathies in current or former MDMA users. Absent studies on point, it may be speculated that as with other 5-HT2B agonists, development of heart valve damage may be dependent on the frequency and duration of use and the total cumulative exposure over time. If that is the case, then the heaviest users are likely to face the greatest risk of heart damage.
Other serotonergic pharmacologics in question
The SSRI antidepressants raise blood serotonin levels , and thus may be capable of the same risks, though it is thought that the risk is substantially lower with such drugs. The amino acid L-tryptophan also raises blood serotonin, and may present the same risk as well; though, again, the risk is considered to be low.
However, the tryptophan derivative 5-HTP (5-hydroxytryptophan), used in the treatment of depression, raises blood serotonin level considerably. It has yet to be reported to be associated with valve disease or other fibrosis, but for the previous theoretical reasons, it has been suggested as a possible danger.
When 5-HTP is used in medicine, it is generally administered along with carbidopa,[29][30] which prevents the peripheral decarboxylation of 5-HTP to serotonin and so ensures that only brain serotonin levels are increased without producing peripheral side effects, however 5-HTP is also sold without carbidopa as a dietary supplement, and may have increased risks when taken by itself without carbidopa.
In non-human great apes
Cardiac fibrosis is common in non-human great apes in human care. The term idiopathic myocardial fibrosis was coined to emphasize this disease is likely different from the above described forms of cardiac fibrosis in humans. The etiology is not known, though vitamin D deficiency is a potential suspected cause at least in chimpanzees.[31]
Possible treatments
The most obvious treatment for cardiac valve fibrosis or fibrosis in other locations, consists of stopping the stimulatory drug or production of serotonin. In the case of a functional neuroendocrine tumor, somatostatin analogs such as octreotide are used to reduce the production of serotonin by tumor cells, which often highly express inhibitory somatostatin receptors.
Surgical tricuspid valve replacement, sometimes combined with a pulmonary valve replacement, can be necessary in some patients.[32]
A compound found in red wine, resveratrol has been found to slow the development of cardiac fibrosis.[33][34][35] More sophisticated approaches of countering cardiac fibrosis like microRNA inhibition (miR-21, for example) are being tested in animal models.
References
- ↑ Gourdie RG, Dimmeler S, Kohl P (September 2016). "Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease". Nature Reviews. Drug Discovery. 15 (9): 620–638. doi:10.1038/nrd.2016.89. PMC 5152911. PMID 27339799.
- 1 2 Chute, Michael; Aujla, Preetinder; Jana, Sayantan; Kassiri, Zamaneh (2019). "The Non-Fibrillar Side of Fibrosis: Contribution of the Basement Membrane, Proteoglycans, and Glycoproteins to Myocardial Fibrosis". Journal of Cardiovascular Development and Disease. 6 (4): 35. doi:10.3390/jcdd6040035. ISSN 2308-3425. PMC 6956278.
- ↑ Gräni, Christoph; Eichhorn, Christian; Bière, Loïc; Kaneko, Kyoichi; Murthy, Venkatesh L.; Agarwal, Vikram; Aghayev, Ayaz; Steigner, Michael; Blankstein, Ron; Jerosch-Herold, Michael; Kwong, Raymond Y. (2019). "Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis". Journal of Cardiovascular Magnetic Resonance. 21 (1). doi:10.1186/s12968-019-0520-0. ISSN 1532-429X. PMC 6393997.
- ↑ Bhaskaran, Ashwin; Tung, Roderick; Stevenson, William G.; Kumar, Saurabh (2019). "Catheter Ablation of VT in Non-Ischaemic Cardiomyopathies: Endocardial, Epicardial and Intramural Approaches". Heart, Lung and Circulation. 28 (1): 84–101. doi:10.1016/j.hlc.2018.10.007. ISSN 1443-9506.
- ↑ Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G (January 2007). "Valvular heart disease and the use of dopamine agonists for Parkinson's disease". The New England Journal of Medicine. 356 (1): 39–46. doi:10.1056/NEJMoa054830. PMID 17202454.
- ↑ Andersohn F, Garbe E (January 2009). "Cardiac and noncardiac fibrotic reactions caused by ergot-and nonergot-derived dopamine agonists". Movement Disorders. 24 (1): 129–33. doi:10.1002/mds.22385. PMID 19170199. S2CID 43196860.
- ↑ Corvol JC, Anzouan-Kacou JB, Fauveau E, Bonnet AM, Lebrun-Vignes B, Girault C, et al. (December 2007). "Heart valve regurgitation, pergolide use, and parkinson disease: an observational study and meta-analysis". Archives of Neurology. 64 (12): 1721–6. doi:10.1001/archneur.64.12.1721. PMID 18071034.
- ↑ Sachdev M, Miller WC, Ryan T, Jollis JG (December 2002). "Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies". American Heart Journal. 144 (6): 1065–73. doi:10.1067/mhj.2002.126733. PMID 12486432.
- ↑ Hopkins PN, Polukoff GI (June 2003). "Risk of valvular heart disease associated with use of fenfluramine". BMC Cardiovascular Disorders. 3: 5. doi:10.1186/1471-2261-3-5. PMC 194859. PMID 12801402.
- ↑ Antonini A, Poewe W (September 2007). "Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease". The Lancet. Neurology. 6 (9): 826–9. doi:10.1016/S1474-4422(07)70218-1. PMID 17706566. S2CID 39526238.
- ↑ Hensrud DD, Connolly HM, Grogan M, Miller FA, Bailey KR, Jensen MD (December 1999). "Echocardiographic improvement over time after cessation of use of fenfluramine and phentermine". Mayo Clinic Proceedings. 74 (12): 1191–7. doi:10.4065/74.12.1191. PMID 10593346.
- ↑ Fleming RM, Boyd LB (2007). "The longitudinal effects of fenfluramine-phentermine use". Angiology. 58 (3): 353–9. doi:10.1177/0003319707302496. PMID 17626991. S2CID 25249333.
- ↑ Baskin, Steven I (1991-09-23). Principles of Cardiac Toxicology. ISBN 9780849388095.
- ↑ Elangbam CS (October 2010). "Drug-induced valvulopathy: an update". Toxicologic Pathology. 38 (6): 837–48. CiteSeerX 10.1.1.1000.286. doi:10.1177/0192623310378027. PMID 20716786. S2CID 20796556.
- ↑ Huang XP, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, et al. (October 2009). "Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment". Molecular Pharmacology. 76 (4): 710–22. doi:10.1124/mol.109.058057. PMC 2769050. PMID 19570945.
- ↑ ADRAC (August 2004). "Cardiac valvulopathy with pergolide". Aust Adv Drug React Bull. 23 (4). Archived from the original on 2012-06-27. Free full text Archived 2007-12-15 at the Wayback Machine from the Australian Therapeutic Goods Administration
- ↑ "MedWatch - 2007 Safety Information Alerts. Permax (pergolide) and generic equivalents". U.S. Food and Drug Administration. March 29, 2007. Retrieved 2007-03-30.
- ↑ Log In Problems
- ↑ Jähnichen S, Horowski R, Pertz H. ""Pergolide and Cabergoline But not Lisuride Exhibit Agonist Efficacy at Serotonin 5-HT2B Receptors"" (PDF). (515 KiB) Presentation. Retrieved on 2007-03-30.
- ↑ Hofmann C, Penner U, Dorow R, Pertz HH, Jähnichen S, Horowski R, et al. (2006). "Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports supports the concept of a crucial role for 5-HT2B receptor agonism in cardiac valvular fibrosis". Clinical Neuropharmacology. 29 (2): 80–6. doi:10.1097/00002826-200603000-00005. PMID 16614540. S2CID 33849447.
- ↑ Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (January 2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- ↑ Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, et al. (June 2003). "3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro". Molecular Pharmacology. 63 (6): 1223–9. doi:10.1124/mol.63.6.1223. PMID 12761331.
- ↑ Schuhmacher M (2007). "[Chiral arylmethoxytryptamines as 5-HT2B-receptor antagonists: synthesis, analysis and in-vitro pharmacology] (German)" (PDF). Ph.D. Dissertation. University of Regensburg: 6–17. Retrieved 2008-08-11.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE (December 1998). "A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor". Journal of Medicinal Chemistry. 41 (26): 5148–9. doi:10.1021/jm9803525. PMID 9857084.
- ↑ Gatch MB (August 2003). "Discriminative stimulus effects of m-chlorophenylpiperazine as a model of the role of serotonin receptors in anxiety". Life Sciences. 73 (11): 1347–67. doi:10.1016/S0024-3205(03)00422-3. PMID 12850497.
- ↑ Nunes-de-Souza V, Nunes-de-Souza RL, Rodgers RJ, Canto-de-Souza A (February 2008). "5-HT2 receptor activation in the midbrain periaqueductal grey (PAG) reduces anxiety-like behaviour in mice". Behavioural Brain Research. 187 (1): 72–9. doi:10.1016/j.bbr.2007.08.030. PMID 17935799. S2CID 25222128.
- ↑ Thomas DR, Gager TL, Holland V, Brown AM, Wood MD (June 1996). "m-Chlorophenylpiperazine (mCPP) is an antagonist at the cloned human 5-HT2B receptor". NeuroReport. 7 (9): 1457–60. doi:10.1097/00001756-199606170-00002. PMID 8856697.
- ↑ Droogmans S, Cosyns B, D'haenen H, Creeten E, Weytjens C, Franken PR, et al. (November 2007). "Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease". The American Journal of Cardiology. 100 (9): 1442–5. doi:10.1016/j.amjcard.2007.06.045. PMID 17950805.
- ↑ Magnussen I, Van Woert MH (1982). "Human pharmacokinetics of long term 5-hydroxytryptophan combined with decarboxylase inhibitors". European Journal of Clinical Pharmacology. 23 (1): 81–6. doi:10.1007/BF01061381. PMID 6182005. S2CID 27371084.
- ↑ Genazzani AR, Sandrini G, Facchinetti F, Rizzo V, Alfonsi E, Sances G, et al. (September 1986). "Effects of L-5HTP with and without carbidopa on plasma beta-endorphin and pain perception: possible implications in migraine prophylaxis". Cephalalgia. 6 (3): 175–9. doi:10.1046/j.1468-2982.1986.0603175.x. PMID 2945645. S2CID 44768866.
- ↑ Strong, Victoria; Moittié, Sophie; Sheppard, Mary N.; Liptovszky, Matyas; White, Kate; Redrobe, Sharon; Cobb, Malcolm; Baiker, Kerstin (2020-01-01). "Idiopathic Myocardial Fibrosis in Captive Chimpanzees (Pan troglodytes)". Veterinary Pathology. 57 (1): 183–191. doi:10.1177/0300985819879442. ISSN 0300-9858.
- ↑ Nguyen A, Schaff HV, Abel MD, Luis SA, Lahr BD, Halfdanarson TR, Connolly HM (July 2019). "Improving outcome of valve replacement for carcinoid heart disease". The Journal of Thoracic and Cardiovascular Surgery. 158 (1): 99–107.e2. doi:10.1016/j.jtcvs.2018.09.025. PMID 30527716.
- ↑ Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG (March 2005). "Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol". American Journal of Physiology. Heart and Circulatory Physiology. 288 (3): H1131-8. doi:10.1152/ajpheart.00763.2004. PMID 15498824.
- ↑ Aubin MC, Lajoie C, Clément R, Gosselin H, Calderone A, Perrault LP (June 2008). "Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol". The Journal of Pharmacology and Experimental Therapeutics. 325 (3): 961–8. doi:10.1124/jpet.107.135061. PMID 18356487. S2CID 10178065.
- ↑ Sutra T, Oiry C, Azay-Milhau J, Youl E, Magous R, Teissèdre PL, et al. (December 2008). "Preventive effects of nutritional doses of polyphenolic molecules on cardiac fibrosis associated with metabolic syndrome: involvement of osteopontin and oxidative stress". Journal of Agricultural and Food Chemistry. 56 (24): 11683–7. doi:10.1021/jf802357g. PMID 19049292.