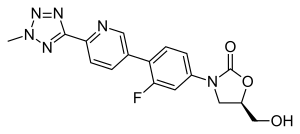
Tedizolid
 | |
| Names | |
|---|---|
| Trade names | Sivextro |
| Other names | TR-700, torezolid |
IUPAC name
| |
| Clinical data | |
| Drug class | Oxazolidinone antibiotic[1] |
| Main uses | Skin and skin structure infection[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614038 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 91% |
| Protein binding | 70–90% |
| Elimination half-life | 12 hours |
| Excretion | Feces |
| Chemical and physical data | |
| Formula | C17H15FN6O3 |
| Molar mass | 370.344 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tedizolid, sold under the brand name Sivextro, is an antibiotic used for skin and skin structure infection including cellulitis and skin abscesses.[2][1] This includes cases due to meticillin-resistant Staphylococcus aureus (MRSA).[1] It can be given by mouth or by gradual injection into a vein.[2]
Common side effects include nausea, headache, diarrhea, and vomiting.[1] Other side effects may include Clostridioides difficile infection.[2] Safety in pregnancy and breastfeeding is unclear.[2] It is in the oxazolidinone class of medications and works by blocking bacteria from making protein.[1]
Tedizolid was approved for medical use in the United States in 2014 and Canada and Europe in 2015.[1][2][4] It is on the World Health Organization's List of Essential Medicines as an alternative to linezolid.[5] In the United States a 6 day course of treatment costs about 2,300 USD as of 2021.[6] This amount in the United Kingdom costs the NHS about £862.[7]
Medical uses
Tedizolid is used for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains, MRSA, and methicillin-susceptible strains), various Streptococcus species (S. pyogenes, S. agalactiae, and S. anginosus group including S. anginosus, S. intermedius, and S. constellatus), and Enterococcus faecalis.[8][9][10][3][1]
Dosage
The recommended dosage for treatment is 200 mg once daily for a total duration of six days, either by mouth (with or without food) or through an intravenous injection (if patient is older than 18 years old).[3]
Side effects
The most common side effects found in the clinical trials were nausea, headache, diarrhea, vomiting, and dizziness.[3] Tedizolid has also been found to have hematologic (blood) effects, as shown in Phase-I studies in which subjects exposed to doses longer than 6 days showed a possible dose and duration effect on hematologic parameters.[3] Its safety in patients with decreased levels of white blood cells has not been established.[10] Patients on tedizolid are also at low risk of peripheral and optic neuropathy, similar to other members of the oxazolidinone class.[3]
Mechanism of action
Tedizolid phosphate (TR-701) is a prodrug activated by plasma or intestinal phosphatases to tedizolid (TR-700) following administration of the drug either orally or intravenously.[3][11] Once activated, tedizolid exerts its bacteriostatic microbial activity through inhibition of protein synthesis by binding to the 50S ribosomal subunit (on the acceptor site) of the bacteria.[3]
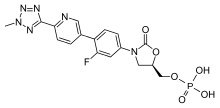
Chemistry
Tedizolid is a second-generation oxazolidinone derivative that is 4-to-16-fold more potent against staphylococci and enterococci compared to linezolid.[12]
History
Tedizolid phosphate is a phosphate ester prodrug of the active compound tedizolid. It was developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics (originator: Dong-A Pharmaceuticals).[13]
Tedizolid is the second treatment approved by the FDA under the new federal law Generating Antibiotic Incentives Now (known as the GAIN Act).[14][15] New antibiotics manufactured under this new act will be designed as a Qualified Infectious Disease Product (QIDP), allowing an expedited review by the FDA and an additional five years of market exclusivity.[15]
Research
Tedizolid showed noninferiority to linezolid in two phase-III trials, known as the ESTABLISH trials.[16]
References
- 1 2 3 4 5 6 7 8 "Sivextro EPAR". European Medicines Agency (EMA). Archived from the original on 8 July 2020. Retrieved 5 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 4 5 6 "Tedizolid Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 24 September 2021.
- 1 2 3 4 5 6 7 8 "Sivextro- tedizolid phosphate tablet, film coated Sivextro- tedizolid phosphate injection, powder, lyophilized, for solution". DailyMed. 22 June 2020. Archived from the original on 26 October 2020. Retrieved 24 October 2020.
- ↑ Canada, Health (4 May 2016). "Health Canada New Drug Authorizations: 2015 Highlights". www.canada.ca. Archived from the original on 20 February 2020. Retrieved 24 September 2021.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Tedizolid Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 7 June 2016. Retrieved 24 September 2021.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 607. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Drug Approval Package: Sivextro (tedizolid phosphate) Tablets NDA #205435". U.S Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 6 July 2020. Retrieved 5 July 2020.
- ↑ "Drug Approval Package: Sivextro (tedizolid phosphate) Injection NDA #205436". U.S Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 6 July 2020. Retrieved 5 July 2020.
- 1 2 "FDA approves Sivextro to treat skin infections" (Press release). June 2014. Archived from the original on 2017-01-21. Retrieved 2019-12-16.
- ↑ Schaadt R, Sweeney D, Shinabarger D, Zurenko G (August 2009). "In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent". Antimicrobial Agents and Chemotherapy. 53 (8): 3236–9. doi:10.1128/AAC.00228-09. PMC 2715649. PMID 19528279.
- ↑ "Tedizolid (TR-701): a new oxazolidinone with enhanced potency" Archived 2021-08-29 at the Wayback Machine. Accessed 2015-03-16.
- ↑ "Cubist Pharmaceuticals to Acquire Trius Therapeutics". July 2013. Archived from the original on 2015-04-02. Retrieved 2021-01-10.
- ↑ "New FDA task force will support innovation in antibacterial drug development". September 2012. Archived from the original on 2017-01-18. Retrieved 2021-01-10.
- 1 2 "Three encouraging steps towards new antibiotics". September 2014. Archived from the original on 2015-03-07. Retrieved 2021-01-10.
- ↑ "Analysis of the Phase 3 ESTABLISH Trials of Tedizolid versus Linezolid in Acute Bacterial Skin and Skin Structure Infections" Archived 2021-03-12 at the Wayback Machine. Accessed March 16, 2015
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Tedizolid phosphate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-27. Retrieved 2021-01-10.
- "Tedizolid Injection: MedlinePlus Drug Information". MedlinePlus. Archived from the original on 2021-01-20. Retrieved 2021-01-10.