Dehydroepiandrosterone sulfate
Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex.[1] It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA.[2] The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
17-Oxoandrost-5-en-3β-yl hydrogen sulfate | |
| Systematic IUPAC name
(3aS,3bR,7S,9aR,9bS,11aS)-9a,11a-Dimethyl-1-oxo-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-yl hydrogen sulfate | |
| Other names
Androstenolone sulfate; Prasterone sulfate; Androst-5-en-3β-ol-17-one 3β-sulfate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DHEA sulfate; DHEA-S; DHEAS |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H28O5S | |
| Molar mass | 368.49 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biological activity
Neurosteroid activity
Similarly to other conjugated steroids, DHEA-S is devoid of hormonal activity, lacking affinity for the steroid hormone receptors.[3][4] However, DHEA-S retains activity as a neurosteroid and neurotrophin.[2] It has been found to act as a positive allosteric modulator of the NMDA receptor (50 nM–1 μM), negative allosteric modulator of the GABAA and glycine receptors, and weak agonist of the sigma-1 receptor (Kd > 50 μM).[2][5] In addition, DHEA-S has been found to directly bind to and activate the TrkA and p75NTR – receptors of neurotrophins like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) – with high affinity (around 5 nM).[2][6][7][8]
Hormonal activity
Although DHEA-S itself is hormonally inert, it has been thought that it can be converted back into DHEA,[9] which is weakly androgenic and estrogenic, and that DHEA in turn can be transformed into more potent androgens like testosterone and dihydrotestosterone (DHT) as well as estrogens like estradiol.[2][1][10] As such, it has been thought that DHEA-S is a prohormone with the potential for androgenic and estrogenic effects.[2][1][10] However, a 2005 study found that DHEA could be converted into DHEA-S but found no evidence of conversion of DHEA-S into DHEA.[11]
Other activity
DHEA-S has also been found to inhibit the TRPV1 and TRPC5 transient receptor potential channels and to inhibit the P2X receptor.[5]
Biochemistry
Biosynthesis
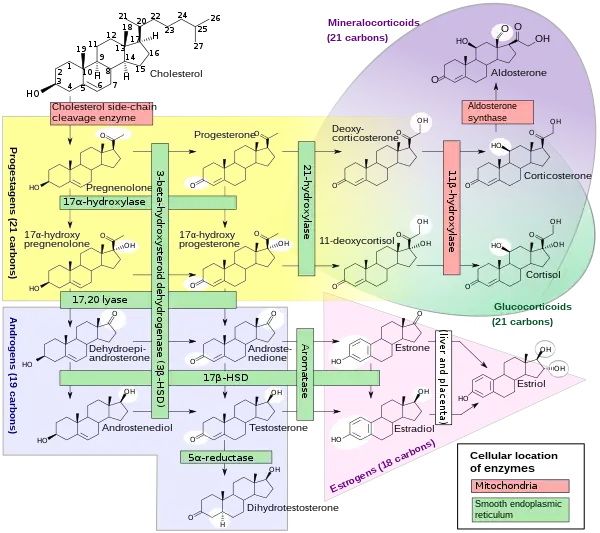
DHEA and DHEA-S are produced in the zona reticularis of the adrenal cortex under the control of adrenocorticotropic hormone (ACTH).[1] DHEA is synthesized from cholesterol via the enzymes cholesterol side-chain cleavage enzyme (CYP11A1; P450scc) and 17α-hydroxylase/17,20-lyase (CYP17A1), with pregnenolone and 17α-hydroxypregnenolone as intermediates.[13] Then, DHEA-S is formed by sulfation of DHEA at the C3β position via the sulfotransferase enzymes SULT2A1 and to a lesser extent SULT1E1.[13][14][15] Whereas DHEA is derived mostly from the adrenal cortex but is also secreted to a lesser extent by the gonads (10%),[16] DHEA-S is almost exclusively produced and secreted by the adrenal cortex, with 95 to 100% originating from the adrenal cortex in women.[1][17][18] Approximately 10 to 15 mg of DHEA-S is secreted by the adrenal cortex per day in young adults.[19]
Distribution
Unlike DHEA, which is weakly bound to albumin, DHEA-S is strongly bound to albumin (i.e., with very high affinity), and this is the reason for its much longer comparative terminal half-life.[20][21] In contrast to DHEA, DHEA-S is not bound to any extent to sex hormone-binding globulin (SHBG).[22]
Whereas DHEA easily crosses the blood–brain barrier into the central nervous system,[23] DHEA-S poorly crosses the blood–brain barrier.[24]
Metabolism
DHEA-S can be converted back into DHEA via steroid sulfatase (STS).[9] In premenopausal women, 40 to 75% of circulating testosterone is derived from peripheral metabolism of DHEA-S, and in postmenopausal women, over 90% of estrogens, mainly estrone, are derived from peripheral metabolism of DHEA-S.[2] A study found that administration of exogenous DHEA-S in women who were pregnant increased circulating levels of estrone and estradiol.[25] DHEA-S serves as a depot for potent androgens like testosterone and dihydrotestosterone in prostate cancer, which fuel the growth of this cancer.[26]
The elimination half-life of DHEA-S is 7 to 10 hours, which is far longer than that of DHEA, which has an elimination half-life of only 15 to 30 minutes.[21]
Levels
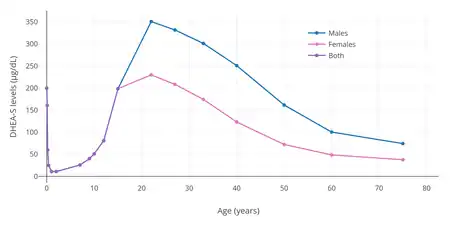
DHEA and DHEA-S are the most abundant circulating steroids in the body.[29] Plasma levels of DHEA-S are 100 or more times higher than those of DHEA, 5 to 10 times higher than those of cortisol, 100 to 500 times those of testosterone, and 1,000 to 10,000 times higher than those of estradiol.[30][3]
Levels of DHEA and DHEA-S vary throughout life.[2][1] They remain low during childhood until adrenarche around 6 to 8 years of age, at which point they markedly increase,[31] eventually peaking at around 20 to 30 years of age.[2][1] From the third decade of life on, DHEA and DHEA-S levels gradually decrease.[29] By the age of 70, levels of DHEA and DHEA-S are 80 to 85% lower than those of young adults, and in people more than 80 years of age, DHEA and DHEA-S levels can reach 80 to 90% lower than those of younger individuals.[29]
Reference ranges
| Tanner stage and average age | Lower limit | Upper limit | Unit | |
|---|---|---|---|---|
| Tanner stage I | >14 days | 16 | 96 | μg/dL |
| Tanner stage II | 10.5 years | 22 | 184 | |
| Tanner stage III | 11.6 years | <15 | 296 | |
| Tanner stage IV | 12.3 years | 17 | 343 | |
| Tanner stage V | 14.5 years | 44 | 332 | |
| 18–29 years | 44 | 332 | ||
| 30–39 years | 31 | 228 | ||
| 40–49 years | 18 | 244 | ||
| 50–59 years | <15 | 200 | ||
| > or =60 years | <15 | 157 | ||
| Tanner stage and average age | Lower limit | Upper limit | Unit | |
|---|---|---|---|---|
| Tanner stage I | >14 days | <15 | 120 | μg/dL |
| Tanner stage II | 11.5 years | <15 | 333 | |
| Tanner stage III | 13.6 years | <15 | 312 | |
| Tanner stage IV | 15.1 years | 29 | 412 | |
| Tanner stage V | 18.0 years | 89 | 457 | |
| 18–29 years | 89 | 457 | ||
| 30–39 years | 65 | 334 | ||
| 40–49 years | 48 | 244 | ||
| 50–59 years | 35 | 179 | ||
| > or =60 years | 25 | 131 | ||
Medical use
Deficiency
The Endocrine Society recommends against the therapeutic use of DHEA-S in both healthy women and those with adrenal insufficiency, as its role is not clear from studies performed so far.[33] The routine use of DHEA-S and other androgens is discouraged in the treatment of women with low androgen levels due to hypopituitarism, adrenal insufficiency, menopause due to ovarian surgery, glucocorticoid use, or other conditions associated with low androgen levels; this is because there are limited data supporting improvement in signs and symptoms with therapy and no long-term studies of risk.[33]
In otherwise elderly women, in whom an age-related fall of DHEA-S may be associated with menopausal symptoms and reduced libido, DHEA-S supplementation cannot currently be said to improve outcomes.[34]
Childbirth
As the sodium salt, prasterone sodium sulfate, DHEA-S is used as a pharmaceutical drug in Japan in the treatment of insufficient cervical ripening and cervical dilation during childbirth.[35][36][37][38][39][40][41]
Diagnostic use
DHEA-S levels above 1890 μM or 700 to 800 μg/dL are highly suggestive of adrenal dysfunction because DHEA-S is made by the adrenal glands[42][43] and also synthesized in the brain.[44] The presence of DHEA-S is therefore used to rule out ovarian or testicular origin of excess androgen.
Women with hirsutism commonly present with mildly elevated DHEA-S levels.[45] Common etiologies for hirsutism include ovarian dysfunction (polycystic ovary syndrome) and adrenal dysfunction (congenital adrenal hyperplasia, cushing's syndrome, androgen secreting tumors); 90% of these cases are caused by PCOS or are idiopathic in nature.[45] However, severely increased DHEA-S levels (>700 μg/dL) necessitate further workup and are almost stem from benign or malignant adrenal alterations.[45]
Chemistry
DHEA-S, also known as androst-5-en-3β-ol-17-one 3β-sulfate, is a naturally occurring androstane steroid and the C3β sulfate ester of DHEA.
References
- Risto Erkkola (2006). The Menopause. Elsevier. pp. 5–. ISBN 978-0-444-51830-9.
- Prough RA, Clark BJ, Klinge CM (2016). "Novel mechanisms for DHEA action". J. Mol. Endocrinol. 56 (3): R139–55. doi:10.1530/JME-16-0013. PMID 26908835.
- Walter K.H. Krause (30 November 2008). Cutaneous Manifestations of Endocrine Diseases. Springer Science & Business Media. pp. 79–. ISBN 978-3-540-88367-8.
Plasma DHEA-S levels in adult men and women are 100-500 times higher than those of testosterone and 1000-10000 times higher than those of estradiol.
- Mo Q, Lu SF, Simon NG (2006). "Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity". J. Steroid Biochem. Mol. Biol. 99 (1): 50–8. doi:10.1016/j.jsbmb.2005.11.011. PMID 16524719. S2CID 30489004.
- Steven R. King (9 November 2012). Neurosteroids and the Nervous System. Springer Science & Business Media. pp. 1, 12. ISBN 978-1-4614-5559-2.
- Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A (2011). "Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis". PLOS Biol. 9 (4): e1001051. doi:10.1371/journal.pbio.1001051. PMC 3082517. PMID 21541365.
- Pediaditakis I, Iliopoulos I, Theologidis I, Delivanoglou N, Margioris AN, Charalampopoulos I, Gravanis A (2015). "Dehydroepiandrosterone: an ancestral ligand of neurotrophin receptors". Endocrinology. 156 (1): 16–23. doi:10.1210/en.2014-1596. PMID 25330101.
- Gravanis A, Calogeropoulou T, Panoutsakopoulou V, Thermos K, Neophytou C, Charalampopoulos I (2012). "Neurosteroids and microneurotrophins signal through NGF receptors to induce prosurvival signaling in neuronal cells". Sci Signal. 5 (246): pt8. doi:10.1126/scisignal.2003387. PMID 23074265. S2CID 26914550.
- Robert Morfin (2 September 2003). DHEA and the Brain. CRC Press. pp. 28–. ISBN 978-0-203-30121-0.
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 545–. ISBN 978-1-4511-4847-3.
- Hammer F, Subtil S, Lux P, Maser-Gluth C, Stewart PM, Allolio B, Arlt W (2005). "No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies". J. Clin. Endocrinol. Metab. 90 (6): 3600–5. doi:10.1210/jc.2004-2386. PMID 15755854.
- Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- Rainey WE, Nakamura Y (February 2008). "Regulation of the adrenal androgen biosynthesis". J. Steroid Biochem. Mol. Biol. 108 (3–5): 281–86. doi:10.1016/j.jsbmb.2007.09.015. PMC 2699571. PMID 17945481.
- Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr. Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- Lawrence H Lash (2005). Drug Metabolism and Transport: Molecular Methods and Mechanisms. Springer Science & Business Media. pp. 353–. ISBN 978-1-59259-832-8.
- Wolf-Bernhard Schill; Frank H. Comhaire; Timothy B. Hargreave (26 August 2006). Andrology for the Clinician. Springer Science & Business Media. pp. 243–. ISBN 978-3-540-33713-3.
- Gretchen M. Lentz; Rogerio A. Lobo; David M. Gershenson; Vern L. Katz (2012). Comprehensive Gynecology. Elsevier Health Sciences. pp. 850–. ISBN 978-0-323-06986-1.
- Dimitrios A. Linos; Jon A. van Heerden (5 December 2005). Adrenal Glands: Diagnostic Aspects and Surgical Therapy. Springer Science & Business Media. pp. 161–. ISBN 978-3-540-26861-1.
- G.A.W. Rook; S. Lightman (6 December 2012). Steroid Hormones and the T-Cell Cytokine Profile. Springer Science & Business Media. pp. 205–. ISBN 978-1-4471-0931-0.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 712–. ISBN 978-0-7817-1750-2.
- Bruce Alan White; Susan P. Porterfield (2013). Endocrine and Reproductive Physiology, Mosby Physiology Monograph Series (with Student Consult Online Access),4: Endocrine and Reproductive Physiology. Elsevier Health Sciences. pp. 164–. ISBN 978-0-323-08704-9.
- Paul M. Coates; M. Coates Paul; Marc Blackman; Marc R. Blackman, Gordon M. Cragg, Mark Levine, Jeffrey D. White, Joel Moss, Mark A. Levine (29 December 2004). Encyclopedia of Dietary Supplements (Print). CRC Press. pp. 170–. ISBN 978-0-8247-5504-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Joseph E. Pizzorno (2013). Textbook of Natural Medicine. Elsevier Health Sciences. pp. 711–. ISBN 978-1-4377-2333-5.
- Samuel S. C. Yen; Robert B. Jaffe; Robert L. Barbieri (January 1999). Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Saunders. p. 40. ISBN 978-0-7216-6897-0.
Thus, the formation of DHEA-S occurs directly in the brain, particularly because DHEA-S does not cross the blood-brain barrier [...]
- Nguyen AD, Conley AJ (2008). "Adrenal androgens in humans and nonhuman primates: production, zonation and regulation". Endocr Dev. Endocrine Development. 13: 33–54. doi:10.1159/000134765. ISBN 978-3-8055-8580-4. PMID 18493132.
- Penning TM (2018). "Dehydroepiandrosterone (DHEA)-SO4 Depot and Castration-Resistant Prostate Cancer". Vitam. Horm. Vitamins and Hormones. 108: 309–331. doi:10.1016/bs.vh.2018.01.007. ISBN 9780128143612. PMC 6226251. PMID 30029732.
- S.S. Nussey; S.A. Whitehead (8 April 2013). Endocrinology: An Integrated Approach. CRC Press. pp. 158–. ISBN 978-0-203-45043-7.
- Mark A. Sperling (10 April 2014). Pediatric Endocrinology E-Book. Elsevier Health Sciences. pp. 485–. ISBN 978-1-4557-5973-6.
- Philip E. Harris; Pierre-Marc G. Bouloux (24 March 2014). Endocrinology in Clinical Practice, Second Edition. CRC Press. pp. 521–. ISBN 978-1-84184-952-2.
- Abraham Weizman (1 February 2008). Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment. Springer Science & Business Media. pp. 261–. ISBN 978-1-4020-6854-6.
- Douglas T. Carrell; C. Matthew Peterson (23 March 2010). Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice. Springer Science & Business Media. pp. 158–. ISBN 978-1-4419-1436-1.
- Dehydroepiandrosterone Sulfate (DHEA-S), Serum Archived 2018-03-14 at the Wayback Machine at Mayo Foundation For Medical Education And Research. Retrieved July 2012
- Wierman, Margaret E.; Arlt, Wiebke; Basson, Rosemary; Davis, Susan R.; Miller, Karen K.; Murad, Mohammad H.; Rosner, William; Santoro, Nanette (2014). "Androgen Therapy in Women: A Reappraisal: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology & Metabolism. 99 (10): 3489–510. doi:10.1210/jc.2014-2260. PMID 25279570.
- Elraiyah, Tarig; Sonbol, Mohamad Bassam; Wang, Zhen; Khairalseed, Tagwa; Asi, Noor; Undavalli, Chaitanya; Nabhan, Mohammad; Altayar, Osama; Prokop, Larry; Montori, Victor M.; Murad, Mohammad Hassan (2014). "The Benefits and Harms of Systemic Dehydroepiandrosterone (DHEA) in Postmenopausal Women With Normal Adrenal Function: A Systematic Review and Meta-analysis". The Journal of Clinical Endocrinology & Metabolism. 99 (10): 3536–42. doi:10.1210/jc.2014-2261. PMC 5393492. PMID 25279571.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–. ISBN 978-1-4757-2085-3.
- John W. Blunt; Murray H. G. Munro (19 September 2007). Dictionary of Marine Natural Products with CD-ROM. CRC Press. pp. 1075–. ISBN 978-0-8493-8217-8.
- A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 2441–2442. ISBN 978-3-13-179525-0.
- Martin Negwer; Hans-Georg Scharnow (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 1831. ISBN 978-3-527-30247-5.
3β-Hydroxyandrost-5-en-17-one hydrogen sulfate = (3β)-3-(Sulfooxy)androst-5-en-17-one. R: Sodium salt (1099-87-2). S: Astenile, Dehydroepiandrosterone sulfate sodium, DHA-S, DHEAS, KYH 3102, Mylis, PB 005, Prasterone sodium sulfate, Teloin
- Jianqiu Y (1992). "Clinical Application of Prasterone Sodium Sulfate". Chinese Journal of New Drugs. 5: 015.
- Sakaguchi M, Sakai T, Adachi Y, Kawashima T, Awata N (1992). "The biological fate of sodium prasterone sulfate after vaginal administration. I. Absorption and excretion in rats". J. Pharmacobio-Dyn. 15 (2): 67–73. doi:10.1248/bpb1978.15.67. PMID 1403604.
- Sakai, Takanori; Sakaguchi, Minoru; Adachi, Yoshiko; Kawashima, Tsuneo; Awata, Norio (1992). "The Biological Fate of Sodium Prasterone Sulfate after Vaginal Administration II: Distribution after Single and Multiple Administration to Pregnant Rats". 薬物動態. 7 (1): 87–101. doi:10.2133/dmpk.7.87.
- Somani N, Harrison S, Bergfeld WF (2008). "The clinical evaluation of hirsutism". Dermatologic Therapy. 21 (5): 376–91. doi:10.1111/j.1529-8019.2008.00219.x. PMID 18844715. S2CID 34029116.
- "Polycystic Ovarian Syndrome Workup". eMedicine. 25 October 2011. Retrieved 19 November 2011.
- Vaudry, H.; Do Rego, J. L.; Burel, D.; Luu-The, V.; Pelletier, G.; Vaudry, D.; Tsutsui, K. (2011). "Neurosteroid Biosynthesis in the Brain of Amphibians". Frontiers in Endocrinology. 2: 79. doi:10.3389/fendo.2011.00079. PMC 3355965. PMID 22649387.
- Sachdeva, Silonie (2010). "Hirsutism: Evaluation and Treatment". Indian Journal of Dermatology. 55. 1 (1): 3–7. doi:10.4103/0019-5154.60342. PMC 2856356. PMID 20418968.