Naltrexone/bupropion
Naltrexone/bupropion, sold under the brand name Contrave among others, is a fixed-dose combination medication for the management of chronic obesity in adults in combination with a reduced-calorie diet and increased physical activity.[2][4] It contains naltrexone, an opioid antagonist, and bupropion, an aminoketone antidepressant.[2] It is taken by mouth.[2] Both medications have individually shown some evidence of effectiveness in weight loss, and the combination has been shown to have some synergistic effects on weight.[5]
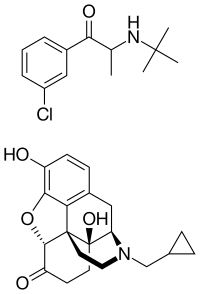 Skeletal structures of bupropion (top) and naltrexone (bottom) | |
| Combination of | |
|---|---|
| Naltrexone | Opioid receptor antagonist |
| Bupropion | Norepinephrine-dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist |
| Clinical data | |
| Trade names | Contrave, Mysimba |
| AHFS/Drugs.com | Monograph |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| KEGG | |
| | |
In September 2014, a sustained release formulation of the drug was approved for marketing in the United States under the brand name Contrave.[6][7] The combination was subsequently approved in the European Union in the spring of 2015, where it is sold under the name Mysimba.[3][8] It was approved in Canada under the Contrave brand name in 2018.[9]
Medical uses
Naltrexone/bupropion is indicated, as an adjunct to a reduced-calorie diet and increased physical activity, as anti-obesity medication for the management of weight in adults with an initial body mass index (BMI) of:[2][3]
- 30 kg/m2 (obese), or[2][3]
- 27 kg/m2 to < 30 kg/m2, (overweight) in the presence of one or more weight-related comorbidities, like type 2 diabetes, dyslipidaemia, or controlled high blood pressure[2][3]
Contraindications
The manufacturer recommends against its use in people that have/are:[2]
- History of seizures
- History of an eating disorder such as bulimia nervosa or anorexia nervosa
- Taking opioid pain medicines, taking medicines to stop opioid addiction, or are in opiate withdrawal
- Taking an MAOI or have taken an MAOI in the last 14 days
- Pregnant
- Abruptly stopped using: alcohol, benzodiazepines, barbiturates, or antiepileptic drugs
Adverse effects
The U.S. Food and Drug Administration (FDA) has put a boxed warning onto this medicine because it may affect mood and increase the likelihood of suicidal thoughts in people under 25 years old.[2] This is attributed to the bupropion component, as antidepressants have been associated with increased risk of suicidal thoughts, but not suicide, and only in people younger than 25.[2]
The safety and effectiveness in children under the age of 18 has not been studied.[2]
Mechanism of action
Individually, naltrexone and bupropion each target pathways in the central nervous system that influence appetite and energy use.
- Bupropion is a reuptake inhibitor and releasing agent of both norepinephrine and dopamine, and a nicotinic acetylcholine receptor antagonist, and it activates proopiomelanocortin (POMC) neurons in the hypothalamus which give an effect downstream, resulting in loss of appetite and increased energy output. The POMC is regulated by endogenous opioids via opioid-mediated negative feedback.
- Naltrexone is a pure opioid antagonist, which further augments bupropion's activation of the POMC.[11]
Combined, naltrexone/bupropion has an effect on the reward pathway that results in reduced food craving.[12] In 2009, Monash University physiologist Michael Cowley was awarded one of Australia's top research honors, the Commonwealth Science Minister's Prize for Life Scientist of the Year, in recognition of his elucidation of these pathways, which led to the development of the combination medication.[13]
History
Orexigen submitted a New Drug Application (NDA) for this drug combination to the U.S. Food and Drug Administration (FDA) on 31 March 2010.[14] Having paid a fee under the Prescription Drug User Fee Act, Orexigen was given a deadline for the FDA to approve or reject the drug of 31 January 2011. On 7 December 2010, an FDA Advisory Committee voted 13-7 for the approval of Contrave, and voted 11-8 for the conduct of a post-marketing cardiovascular outcomes study.[15] Subsequently, on 2 February 2011, the FDA rejected the drug and it was decided that an extremely large-scale study of the long-term cardiovascular effects of Contrave would be needed, before approval could be considered.[16] It was ultimately approved in the United States in the fall of 2014.[7]
In December 2014, the EU's Committee for Medicinal Products for Human Use (CHMP) endorsed the combination for licensure as an obesity medication when used alongside diet and exercise.[17] Approval was granted in late March 2015.[8]
In May 2015, Orexigen ended a safety study of its diet drug earlier than planned, because an independent panel of experts says the drug maker “inappropriately” compromised the trial by prematurely releasing interim data. The early data release reported a reduction in heart attacks that was no longer observed when a more complete view of the data was analyzed.[18]
In 2018, Orexigen sold its assets, including Contrave, to Nalpropion Pharmaceuticals.[19][20]
On 22 September 2020, the FDA issued a Warning Letter to Nalpropion Pharmaceuticals LLC on concerns of a sponsored Google link making "false or misleading claims about the risks associated with and efficacy of Contrave"[21] on multiple issues. Nalproprion subsequently issued "An important correction from CONTRAVE® (naltrexone HCl/bupropion HCl) Extended-Release Tablets" [22]
Marketing and sales
The sustained-release formulation, Contrave, is marketed by Takeda under license from the combination medication's developer, Orexigen Therapeutics.[7] As of 2015, Orexigen received 20% of net sales from Takeda.[23]
At the time of its approval by the FDA, Wells Fargo analyst Matthew Andrews estimated that Contrave's U.S. sales would reach approximately US$200,000,000 in 2016, exceeding that of the dominant alternative obesity medications lorcaserin and phentermine/topiramate.[24] Despite being initially impeded by technical issues, the growth in filled prescriptions in the first months after approval was very rapid — substantially exceeding the equivalent early uptake of either of the two alternative medications just cited.[25] The first quarter of sales for Contrave (Q1 2015) showed net sales of US$11,500,000.[23]
Despite having been approved for use in Europe in March 2015, sales of Contrave have not begun as Orexigen has not yet found a marketing partner.[23]
References
- "Regulatory Decision Summary - Contrave". Health Canada. 23 October 2014. Retrieved 7 June 2022.
- "Contrave Extended-Release- naltrexone hydrochloride and bupropion hydrochloride tablet, extended release". DailyMed. 26 April 2019. Retrieved 5 August 2020.
- "Mysimba EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 5 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Plodkowski RA, Nguyen Q, Sundaram U, Nguyen L, Chau DL, St Jeor S (April 2009). "Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity". Expert Opinion on Pharmacotherapy. 10 (6): 1069–81. doi:10.1517/14656560902775750. PMID 19364254. S2CID 56625956.
- Tek C (2016). "Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: patient selection and perspectives". Patient Preference and Adherence. 10: 751–9. doi:10.2147/PPA.S84778. PMC 4862388. PMID 27217728.
- "Drug Approval Package: Contrave (naltrexone hydrochloride/bupropion hydrochloride) Extended-Release Tablets NDA #200063". U.S. Food and Drug Administration (FDA). 12 November 2014. Retrieved 5 August 2020.
- "FDA approves weight-management drug Contrave" (Press release). FDA. 10 September 2014. Archived from the original on 17 February 2017. Retrieved 16 December 2019.
- Orexigen Therapeutics, Inc. (March 26, 2015). "Orexigen's Mysimba Approved in Europe for the Treatment of Obesity". Yahoo! Finance. PR Newswire. Retrieved 28 March 2015.
- "Regulatory Decision Summary - Contrave - Health Canada". Health Canada. 13 February 2018. Retrieved 5 September 2021.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. (January 2009). "Rational design of a combination medication for the treatment of obesity". Obesity. 17 (1): 30–9. doi:10.1038/oby.2008.461. PMID 18997675. S2CID 24856014.
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. (May 2013). "A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II)". Obesity. 21 (5): 935–43. doi:10.1002/oby.20309. PMC 3739931. PMID 23408728.
- "Obesity expert named Life Scientist of the Year". Monash University. 29 October 2009. Archived from the original on 2 November 2009.
- Orexigen Therapeutics Submits Contrave New Drug Application to FDA for the Treatment of Obesity
- "Press Release". Orexigen Therapeutics, Inc. 2010-12-07. Retrieved 2016-12-29.
- Contrave, Drugs.com
- "Orexigen's weight-loss drug gets thumbs-up from CHMP". Fierce Pharma. 2014-12-19. Retrieved 2016-12-29.
- Silverman E (2015-05-12). "Orexigen Study for Diet Drug Ends Over Premature Data Disclosure". WSJ. Retrieved 2016-12-29.
- "Orexigen, seller of weight-loss drug Contrave, agrees to sale for $75 million". San Diego Union-Tribune. 23 April 2018. Retrieved 5 August 2020.
- "Nalpropion Pharmaceuticals, Inc. Expands Agreement with iNova Pharmaceuticals for Exclusive Commercialization Rights for Contrave (naltrexone HCl / bupropion HCl extended release) to include Select Markets in Southeast Asia, Africa and the Pacific" (Press release). Nalpropion Pharmaceuticals. 19 December 2018. Retrieved 5 August 2020 – via GlobeNewswire.
- "Nalpropion Pharmaceuticals LLC - 09/22/2020". Food and Drug Administration. 23 March 2021.
- https://www.correctiveadvertisementforcontrave.com/
- Osborne S (May 8, 2015). "Orexigen Posts Loss - Revenue Will Be The Story". Seeking Alpha (blog). Retrieved May 9, 2015.
- Grover N (September 10, 2014). "Reuters More: Reuters Health Long-awaited Diet Pill Gets U.S. Approval Reuters". Business Insider. Reuters. Retrieved March 28, 2015.
- Osborne S (December 12, 2014). "Contrave Sales Continue To Impress". Seeking Alpha (blog). Retrieved March 28, 2015.
External links
- "Bupropion mixture with naltrexone". Drug Information Portal. U.S. National Library of Medicine.