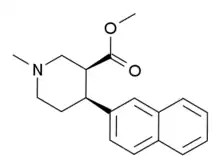
N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
N,O-Dimethyl-4β-(2-naphthyl)piperidine-3β-carboxylate (DMNPC) is a piperidine based stimulant drug which is synthesised from arecoline. It is similar to nocaine in chemical structure, and has two and a half times more activity than cocaine as a dopamine reuptake inhibitor. However it is also a potent serotonin reuptake inhibitor, with similar affinity to fluoxetine.[1]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
| Ki Affinity of Piperidine Based MAT Inhibitors | ||||||
| ∗∗ | X | N | 5HT | DA | NE | |
| SS | p-Vinyl | Me | 138 | 131 | 175 | |
| p-Ethyl | 255 | >1.7K | >1.1K | |||
| p-Allyl | 309 | 964 | >1K | |||
| p-Ethynyl | 175 | 187 | 364 | |||
| p-Phenyl | 62 | 173 | 203 | |||
| β-Naphthyl | 7.6 | 21 | 34 | |||
| 3R,4S | 42 | 947 | 241 | |||
| RR | 192 | 87 | 27 | |||
| 3S,4R | 12 | 271 | 38 | |||
| H2Cl | 3.5 | 90 | 30 | |||
| SS/RR | α-Naphthyl | Me | 101 | 304 | 281 | |
The stereochemistry of DMNPC dramatically affects it's binding affinity with the SS enantiomer having the highest overall activity. The 3R,4S enantiomer demonstrates the highest selectivity for 5-HTT.
In animal studies, DMNPC exhibits similar potency as fluoxetine but with greater activity for DAT and NET. N-Demethylation of DMNPC has shown to produce a 3-fold increase in potency for 5-HTT.[1]
Synthesis
A racemic mixture of DMNPC can be synthesized from freebase arecoline in a grignard reaction with 2-naphthylmagnesium bromide. Further reactions and separation methods can be used to produce enantiomerically pure products.[1]
A substantially simpler method that ablates the carbomethoxy ester substituent has been demonstrated by D. Koch in Ex2: U.S. Patent 6,303,627
See also
References
- Tamiz AP, Zhang J, Flippen-Anderson JL, Zhang M, Johnson KM, Deschaux O, et al. (March 2000). "Further SAR Studies of Piperidine-Based Analogues of Cocaine. 2. Potent Dopamine and Serotonin Reuptake Inhibitors". Journal of Medicinal Chemistry. 43 (6): 1215–22. doi:10.1021/jm9905561. PMID 10737754.