Cilostazol
Cilostazol, sold under the brand name Pletal among others, is a medication used to help the symptoms of intermittent claudication in peripheral vascular disease.[1] If no improvement is seen after 3 months, stopping the medication is reasonable.[2] It may also be used to prevent stroke.[1] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪˈlɒstəzɒl/ sil-OS-tə-zol |
| Trade names | Pletal |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601038 |
| License data |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 95–98% |
| Metabolism | Liver (CYP3A4- and CYP2C19-mediated) |
| Elimination half-life | 11–13 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.897 |
| Chemical and physical data | |
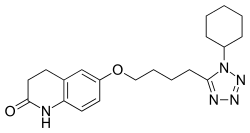
| Formula | C20H27N5O2 |
| Molar mass | 369.469 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include headache, diarrhea, dizziness, and cough.[1] Serious side effects may include decreased survival in those with heart failure, low platelets, and low white blood cells.[1] Cilostazol is a phosphodiesterase 3 inhibitor which works by inhibiting platelet aggregation and dilating arteries.[1]
Cilostazol was approved for medical use in the United States in 1999.[1] It is available as a generic medication.[2] In 2017, it was the 301st most commonly prescribed medication in the United States, with more than one million prescriptions.[3]
Medical uses
Cilostazol is approved for the treatment of intermittent claudication in the United States and United Kingdom.[1][4]
Cilostazol is also used for secondary stroke prevention,[1] though to date no regulatory body has approved it specifically for that indication.
Heart failure
Cilostazol is dangerous for people with severe heart failure. Cilostazol has been studied in people without heart failure, without evidence of harm, but much more data would be needed to determine no risk exists. Although cilostazol would not be approvable for a trivial condition the Cardio-Renal Advisory Committee and FDA concluded that fully informed patients and physicians should be able to choose to use it to treat intermittent claudication. Patient and physician labeling will describe the basis for concern and the incomplete information available.[5]
Adverse effects
Possible side effects of cilostazol use include headache (the most common), diarrhea, severe heat intolerance, abnormal stools, increased heart rate, and palpitations.[6]
Interactions
Cilostazol is metabolized by CYP3A4 and CYP2C19, two isoenzymes of the cytochrome P450 system. Drugs that inhibit CYP3A4, such as itraconazole, erythromycin, ketoconazole, and diltiazem, are known to interact with cilostazol. The proton pump inhibitor omeprazole, an inhibitor of CYP2C19, increases exposure to the active metabolite of cilostazol.[6][7]
A single report has been made of grapefruit juice possibly increasing the effects of cilostazol;[8] some drug information sources list this as a possible interaction.[9][10][11] The FDA-approved labeling of cilostazol notes that grapefruit juice (which is a CYP3A4 inhibitor) increases the drug's maximum concentration by around 50%.[6]
Mechanism
Cilostazol is a selective inhibitor of phosphodiesterase type 3 (PDE3) with therapeutic focus on increasing cAMP. An increase in cAMP results in an increase in the active form of protein kinase A (PKA), which is directly related with an inhibition in platelet aggregation. PKA also prevents the activation of an enzyme (myosin light-chain kinase) that is important in the contraction of smooth muscle cells, thereby exerting its vasodilatory effect.
References
- "Cilostazol Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 23 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 231–232. ISBN 9780857113382.
- "Cilostazol - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- "CILOSTAZOL". BNF. NICE. Retrieved 20 February 2021.
- Center for Drug Evaluation and Research (August 11, 1999). "Approval of Cilostazol". U.S. Food and Drug Administration. Archived from the original on 2007-04-27. Retrieved 2007-04-30.
- "Cilostazol: Official FDA information, side effects and uses". Drugs.com. February 2008. Retrieved 2008-09-22.
- FDA. "Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers". Retrieved 2020-03-25.
- Taniguchi K, Ohtani H, Ikemoto T, Miki A, Hori S, Sawada Y (October 2007). "Possible case of potentiation of the antiplatelet effect of cilostazol by grapefruit juice". Journal of Clinical Pharmacy and Therapeutics. 32 (5): 457–9. doi:10.1111/j.1365-2710.2007.00844.x. PMID 17875111. S2CID 42556945.
- "Cilostazol for peripheral arterial disease". Yahoo! Health. Archived from the original on 2009-10-01. Retrieved 2008-09-21.
- "Cilostazol". MedicineNet.com. May 25, 1999. Retrieved 2008-09-22.
- Cerner-Multum, Inc. (November 29, 2007). "Consumer Drug Information: Cilostazol". Drugs.com. Retrieved 2008-09-22.
External links
- "Cilostazol". Drug Information Portal. U.S. National Library of Medicine.