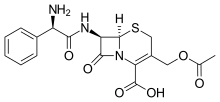
Cefaloglycin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.633 |
| Chemical and physical data | |
| Formula | C18H19N3O6S |
| Molar mass | 405.43 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Cefaloglycin INN (also spelled cephaloglycin) is a first-generation cephalosporin antibiotic.
External links
- Tune B, Hsu C (1990). "The renal mitochondrial toxicity of beta-lactam antibiotics: in vitro effects of cephaloglycin and imipenem". J Am Soc Nephrol. 1 (5): 815–21. doi:10.1681/ASN.V15815. PMID 2133431.
- Tune B, Fravert D, Hsu C (1989). "Oxidative and mitochondrial toxic effects of cephalosporin antibiotics in the kidney. A comparative study of cephaloridine and cephaloglycin". Biochem Pharmacol. 38 (5): 795–802. doi:10.1016/0006-2952(89)90233-5. PMID 2930580.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.