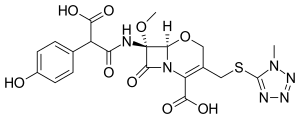
Latamoxef
 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intramuscular, intravenous |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Pharmacokinetics | |
| Protein binding | 35 to 50% |
| Metabolism | Nil |
| Elimination half-life | 2 hours |
| Excretion | Mostly renal, unchanged; also biliary |
| Chemical and physical data | |
| Formula | C20H20N6O9S |
| Molar mass | 520.47 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 117 to 122 °C (243 to 252 °F) (dec.) |
SMILES
| |
InChI
| |
Latamoxef (or moxalactam) is an oxacephem antibiotic. In oxacephems such as latamoxef, the sulfur atom of the cephalosporin core is replaced with an oxygen atom.
It is in the third-generation cephalosporin family of medications and works by interfering with the bacteria's cell wall.[1]
Latamoxef has been associated with prolonged bleeding time, and several cases of coagulopathy, some fatal, were reported during the 1980s.[2][3] Latamoxef is no longer available in the United States. As with other cephalosporins with a methylthiotetrazole side chain, latamoxef causes an antabuse reaction when mixed with alcohol. Additionally, the methylthiotetrazole side chain inhibits γ-carboxylation of glutamic acid; this can interfere with the actions of vitamin K.
It is no longer available in the United States.[1]
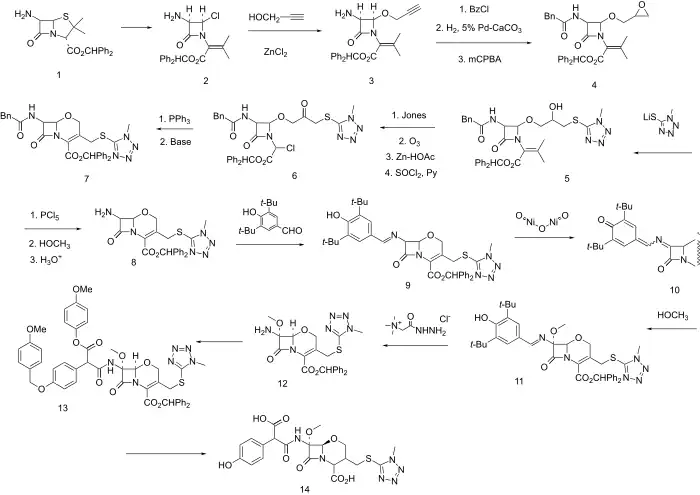
Synthesis
Oxa-substituted third generation cephalosporin antibiotic (oxacephalosporin).
The benzhydrol ester of 6-Aminopenicillanic acid (6-APA) is S-chlorinated and treated with base whereupon the intermediate sulfenyl chloride fragments (to 2). Next, displacement with propargyl alcohol in the presence of zinc chloride gives predominanntly the stereochemistry represented by diastereoisomer 3. The side chain is protected as the phenylacetylamide; the triple bond is partially reduced with a 5% Pd-CaCO3 (Lindlar catalyst) and then epoxidized with mCPBA to give 4. The epoxide is opened at the least hindered end with 1-methyl-1H-tetrazole-5-thiol to put in place the future C-3 side chain and give intermediate 5. Jones oxidation followed in turn by ozonolysis (reductive work-up with zinc-AcOH) and reaction with SOCl2 and pyridine give halide 6. The stage is now wet for intramolecular Wittig reaction. Displacement with PPh3 and Wittig olefination gives 1-oxacephem 7. Next a sequence is undertaken of side chain exchange and introduction of a 7-methoxyl group analogous to that which is present in cephamycins and gives them the enhanced beta-lactamase stability. First 7 is converted to the imino chloride with PCl5 and then to the imino methyl ether (with methanol) and next hydrolyzed to the free amine (8). Imine formation with 3,5-di-t-butyl-4-hydroxybenzaldehyde is next carried out leading to 9. Oxidation with nickel(III) oxide gives iminoquinone methide 10, to which methanol is added in a conjugate sense and in the sterechemistry represented by formula 11. The imine is exchanged with Girard's reagent T to give 12, and this is acylated by a suitable protected arylmalonate, as the hemiester hemiacid chloride so as to give 11. Deblocking with aluminium chloride and anisole gives moxalactam 14.
References
- 1 2 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 832. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-30.
- ↑ Weitekamp MR, Aber RC (1983). "Prolonged bleeding times and bleeding diathesis associated with moxalactam administration". JAMA. 249 (1): 69–71. doi:10.1001/jama.249.1.69. PMID 6217353.
- ↑ Brown RB, Klar J, Lemeshow S, Teres D, Pastides H, Sands M (1986). "Enhanced bleeding with cefoxitin or moxalactam. Statistical analysis within a defined population of 1493 patients". Arch Intern Med. 146 (11): 2159–64. doi:10.1001/archinte.146.11.2159. PMID 3778044.
- ↑ M. Narisada, W. Nagata, DE 2713370; eidem, U.S. Patent 4,138,486 (1977, 1979 both to Shionogi).
- ↑ Narisada, Masayuki; Yoshida, Tadashi; Onoue, Hiroshi; Ohtani, Mitsuaki; Okada, Tetsuo; Tsuji, Teruji; Kikkawa, Ikuo; Haga, Nobuhiro; Satoh, Hisashi; et al. (1979). "Synthetic studies on β-lactam antibiotics. Part 10. Synthesis of 7β-[2-carboxy-2-(4-hydroxyphenyl)acetamido]-7.alpha.-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-1-oxa-1-dethia-3-cephem-4-carboxylic acid disodium salt (6059-S) and its related 1-oxacephems". Journal of Medicinal Chemistry. 22 (7): 757–9. doi:10.1021/jm00193a001. PMID 448673.
- ↑ Otsuka, H.; Nagata, W.; Yoshioka, M.; Narisada, M.; Yoshida, T.; Harada, Y.; Yamada, H. (1981). "Discovery and development of moxalactam (6059-S): The chemistry and biology of 1-oxacephems". Medicinal Research Reviews. 1 (3): 217–48. doi:10.1002/med.2610010302. PMID 6213825. S2CID 45623930.
- ↑ Narisada, Masayuki; Onoue, Hiroshi; Nagata, Wataru (1977). "Synthetic Studies on b-Lactam Antibiotics. Part 5. A Synthesis of 7b-Acylamino-3-methyl-1-oxadethia-3-cephem-4-carboxylic Acids". Heterocycles. 7 (2): 839. doi:10.3987/S-1977-02-0839.
External links
| Identifiers: |
|---|