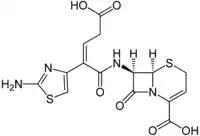
Ceftibuten
 | |
| Names | |
|---|---|
| Trade names | Cedax |
| Other names | Cephtibuten |
IUPAC name
| |
| Clinical data | |
| Drug class | 3rd-generation cephalosporin[1] |
| Main uses | Middle ear infection, strep throat, pneumonia, urinary tract infection[2] |
| Side effects | Nausea, diarrhea, headache, abdominal pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Typical dose | 400 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698023 |
| Chemical and physical data | |
| Formula | C15H14N4O6S2 |
| Molar mass | 410.42 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ceftibuten, sold under the brand name Cedax among others, is an antibiotic used to treat middle ear infection, strep throat, pneumonia, and urinary tract infection.[2] Use is not generally recommended for sinusitis.[2] It is taken by mouth.[2]
Common side effects include nausea, diarrhea, headache, and abdominal pain.[2] Other side effects may include anaphylaxis, Stevens-Johnson syndrome, and Clostridioides difficile infection.[2] While there is no evidence of harm in pregnancy, such use has not been well studied.[3] It is a third-generation cephalosporin and works by interfering with the bacterial cell wall.[1]
Ceftibuten was approved for medical use in the United States in 1995.[2] It has been discontinued in the United States as of 2021.[4]
Medical uses
Ceftibuten is used to treat acute bacterial exacerbations of chronic bronchitis (ABECB), acute bacterial otitis media, pharyngitis, and tonsilitis. It is also indicated for pneumonia, infections of the urinary tract, enteritis, and gastroenteritis.
Dosage
The typical dose is 400 mg per day.[2]
Spectrum of activity
Ceftibuten is active against Haemophilus influenzae, Moraxella catarrhalis, Escherichia coli, Klebsiella pneumoniae, K. oxytoca, Proteus vulgaris, P. mirabilis, P. providence, Salmonella sp., Shigella sp., Enterobacter sp., and Streptococcus sp.
The following represents minimum inhibitory concentration (MIC) susceptibility data for a few clinically significant microorganisms:
- Haemophilus influenzae: 0.015–1.0 μg/ml
- Moraxella catarrhalis: 0.5–4.0 μg/ml
- Streptococcus pneumoniae: 0.5–256 μg/ml [5]
Side effects
The most frequent reactions were gastrointestinal and nausea.
Society and culture
It was marketed by Pernix Therapeutics.
References
- 1 2 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 832. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-30.
- 1 2 3 4 5 6 7 8 9 10 "Ceftibuten Monograph for Professionals". Drugs.com. Archived from the original on 2 August 2019. Retrieved 2 January 2022.
- ↑ "Ceftibuten (Cedax) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 2 January 2022.
- ↑ "Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. Archived from the original on 22 March 2021. Retrieved 2 January 2022.
- ↑ "Ceftibuten Susceptibility and Minimum Inhibitory Concentration Range (MIC) Data" (PDF). TOKU-E. June 2020. Archived (PDF) from the original on 2019-08-02. Retrieved 2021-06-21.
External links
| Identifiers: |
|---|