Sulbactam
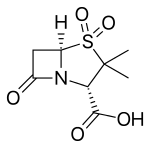 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a693021 |
| Routes of administration | Injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 29% |
| Elimination half-life | 0.65–1.20 hrs |
| Excretion | Mainly kidneys (41–66% within 8 hrs) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.063.506 |
| Chemical and physical data | |
| Formula | C8H11NO5S |
| Molar mass | 233.24 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 148 to 151 °C (298 to 304 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Sulbactam is a β-lactamase inhibitor. This drug is given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys the antibiotics.[1]
It was patented in 1977 and approved for medical use in 1986.[2]
Medical uses
Sulbactam is able to inhibit the most common forms of β-lactamase but is not able to interact with the AmpC cephalosporinase. Thus, it confers little protection against bacteria such as Pseudomonas aeruginosa, Citrobacter, Enterobacter, and Serratia, which often express this gene.
In the United States, sulbactam is combined to form ampicillin/sulbactam. It does possess some antibacterial activity when administered alone, but it is too weak to have any clinical importance. Its use in the UK is restricted to hospitals.
The combination cefoperazone/sulbactam (Sulperazon) is available in many countries.[3]
Recently, its use in treating Acinetobacter sepsis is receiving renewed interest.
Mechanism
Sulbactam is an irreversible inhibitor of β-lactamase; it binds to the enzyme and does not allow it to degrade the antibiotic.
See also
- Sultamicillin, an ester of sulbactam with the penicillin antibiotic ampicillin
- Other betalactamase inhibitors
References
- ↑ Totir MA, Helfand MS, Carey MP, et al. (August 2007). "Sulbactam forms only minimal amounts of irreversible acrylate-enzyme with SHV-1 beta-lactamase". Biochemistry. 46 (31): 8980–7. doi:10.1021/bi7006146. PMC 2596720. PMID 17630699.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 492. ISBN 9783527607495.
- ↑ "Sulperazon". drugs.com.
Further reading
- Singh GS (January 2004). "Beta-lactams in the new millennium. Part-II: cephems, oxacephems, penams and sulbactam". Mini Rev Med Chem. 4 (1): 93–109. doi:10.2174/1389557043487547. PMID 14754446.