Teixobactin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | Unknown |
| Metabolism | Unknown |
| Onset of action | Unknown |
| Elimination half-life | Unknown |
| Excretion | Unknown |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
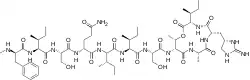
| Formula | C58H95N15O15 |
| Molar mass | 1242.488 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Teixobactin (/ˌteɪksoʊˈbæktɪn/) is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
Teixobactin was discovered using a new method of culturing bacteria in soil, which allowed researchers to grow a previously unculturable bacterium now named Eleftheria terrae, which produces the antibiotic. Teixobactin was shown to kill Staphylococcus aureus and Mycobacterium tuberculosis.
History
In January 2015, a collaboration of four institutes in the US and Germany together with two pharmaceutical companies, reported that they had isolated and characterized a new antibiotic, killing "without detectable resistance."[1][2][3][4][5] Teixobactin was discovered by screening previously unculturable bacteria present in a sample of soil from "a grassy field in Maine,"[5] using the isolation chip (iChip).[6]
The multiple independent iChip culture cells in a block of plastic are inoculated with soil diluted to deposit about one bacterium in each cell, and then sealed with semi-permeable membranes. The iChip is then planted in a box of the soil of origin. Nutrients and growth factors diffusing from the ambient soil into each culture cell through the membranes nurture growth of the bacterium into a colony that is then self-sustaining in vitro. This arrangement allows growth of only one species in some of the cells.[7]
Tests for antibacterial activity against Staphylococcus aureus highlighted a previously undescribed bacterium which was named Eleftheria terrae. It was found to be producing a new antibiotic compound that the researchers named teixobactin.[1] Its absolute stereochemistry was determined employing techniques that included chemical degradation with advanced Marfey's analysis as well as partial degradation, synthesis of fragments obtained by degradation and the synthesis of all four diastereomers of an unusual amino acid not occurring in proteins.[8]
Teixobactin is the first novel antibiotic with drug potential isolated from bacteria in decades. It appears to represent a new class of antibiotics, raising hopes that the new isolation techniques employed could lead to further antibiotic discoveries.[2][4][9][10][11]
Biosynthesis
Teixobactin is an 11-residue, macrocyclic depsipeptide hypothesized by its discoverers to be synthesized in E. terrae by the nonribosomal peptide synthetases Txo1 and Txo2 (encoded by the genes txo1 and txo2).[1] The peptide has several unusual features, including four D-amino acids, a methylated phenylalanine, and the non-proteinogenic α-amino acid enduracididine. The amino acid sequence of teixobactin is MeHN—d-Phe—Ile—Ser——d-Gln—d-Ile—Ile—Ser—d-Thr*—Ala—enduracididine—Ile—COO—*. The carboxy terminus forms a lactone with the l-threonine residue (indicated by the asterisk), as is common in microbial nonribosomal peptides. This lactone-forming ring closure reaction is catalyzed by two C-terminal thioesterase domains of Txo2, forming a lactone.[1] Txo1 and Txo2 are together composed of 11 modules, and each module is thought to sequentially add one amino acid to a growing peptide chain. The first module has a methyltransferase domain that methylates the N-terminal phenylalanine.
Antibacterial activity
Mechanism of action
Teixobactin is an inhibitor of cell wall synthesis. It acts primarily by binding to lipid II, a precursor to peptidoglycan, whereby teixobactin's capacity to form large cluster networks on the bacterial plasma-membrane is decisive. The incorporation of D- and L- amino acids enables allocating teixobactin's hydrophobic residues onto the bacterial membrane surface. [12] Lipid II is also targeted by the antibiotic vancomycin. The binding of teixobactin to lipid precursors inhibits the production of the peptidoglycan layer, leading to lysis of vulnerable bacteria.[1]
Activity
Teixobactin was reported to be potent in vitro against all gram-positive bacteria tested, including Staphylococcus aureus and difficult-to-treat enterococci, with Clostridium difficile and Bacillus anthracis being exceptionally vulnerable. It also killed Mycobacterium tuberculosis. It was also found to be effective in vivo, when used to treat mice infected with methicillin-resistant S. aureus (MRSA), and Streptococcus pneumoniae. The dose required to achieve 50% survival against MRSA is only 10% of the PD50 dose of vancomycin, an antibiotic typically used for MRSA.[1]
It is not active against bacteria with an outer membrane such as gram negative pathogens, particularly carbapenem resistant enterobacteriaceae, or those with New Delhi metallo-beta-lactamase 1 (NDM1).[9]
Induction of resistance
No resistant strain of S. aureus or M. tuberculosis was generated in vitro when administering sublethal doses, for as long as 27 days in the case of the former.[1][3] It is postulated that teixobactin is more robust against mutation of the target pathogens, because of its unusual antibiotic mechanism of binding to less mutable fatty molecules rather than binding to relatively mutable proteins in the bacterial cell.[4] However, several scientists caution that it is too early to conclude that teixobactin resistance would not develop in the clinical setting.[13][14] Similar claims were made for vancomycin, yet resistance emerged soon after large-scale use in the 1980s. It is possible that genes encoding resistance to teixobactin are already present in soil bacteria. Resistance could also arise by mutation after prolonged use in patients.[15]
Society and culture
NovoBiotic Pharmaceuticals has been issued two US patents on teixobactin (US Patents 9,163,065 and 9,402,878). Northeastern University, where Kim Lewis, the lead author of the article in Nature, works, filed a patent on the method used to discover teixobactin, and licensed it to NovoBiotic in 2003; Lewis is a consultant to the company.[5]
Research
In 2018 researchers synthesized the compound and used it to treat a bacterial infection in mice.[16][11]
See also
| Wikimedia Commons has media related to Teixobactin. |
References
- 1 2 3 4 5 6 7 Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K (7 January 2015). "A new antibiotic kills pathogens without detectable resistance". Nature. 517 (7535): 455–9. Bibcode:2015Natur.517..455L. doi:10.1038/nature14098. PMC 7414797. PMID 25561178.
- 1 2 Wright, Gerard (7 January 2015). "Antibiotics: An irresistible newcomer". Nature. 517 (7535): 442–444. Bibcode:2015Natur.517..442W. doi:10.1038/nature14193. PMID 25561172. S2CID 4464402.
- 1 2 Lewis, Kim (7 January 2015). "NovoBiotic reports the discovery of teixobactin, a new antibiotic without detectable resistance" (PDF). Cambridge, Massachusetts: NovoBiotic Pharmaceuticals. Retrieved 7 January 2015.
- 1 2 3 Gallagher, James (7 January 2015). "Antibiotics: US discovery labelled 'game-changer' for medicine". BBC. Retrieved 7 January 2015.
- 1 2 3 Denise, Grady (7 January 2015). "From a pile of dirt, hope for a powerful new antibiotic". The New York Times. Retrieved 7 January 2015.
- ↑ Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Belanger A, Kanigan T, Lewis K, Epstein SS (2010). "Use of ichip for high-throughput in situ cultivation of "uncultivable" microbial species". Appl. Environ. Microbiol. 76 (8): 2445–50. Bibcode:2010ApEnM..76.2445N. doi:10.1128/AEM.01754-09. PMC 2849220. PMID 20173072.
- ↑ Khatchadourian, Raffi (20 June 2016). "The Unseen: Millions of microbes are yet to be discovered. Will one hold the ultimate cure?". The New Yorker. New York: Condé Nast. Retrieved 27 June 2016.
- ↑ Matthews, Andy (8 January 2015). "Selcia Scientists Elucidate Stereochemistry of Novel Antibacterial Macrocycle Teixobactin, Published in Nature". Essex, U.K.: Selcia. Retrieved 10 January 2015.
- 1 2 Judy Stone (8 January 2015). "Teixobactin And iChip Promise Hope Against Antibiotic Resistance". Forbes. Retrieved 10 January 2015.
- ↑ Sample, Ian (8 January 2015). "New class of antibiotic could turn the tables in battle against superbugs". The Guardian. Retrieved 11 January 2015.
- 1 2 Gunjal, Vidya B.; Thakare, Ritesh; Chopra, Sidharth; Reddy, D. Srinivasa (2020). "Teixobactin: A Paving Stone toward a New Class of Antibiotics?". Journal of Medicinal Chemistry. 63 (21): 12171–12195. doi:10.1021/acs.jmedchem.0c00173. ISSN 0022-2623. PMID 32520557.
- ↑ Shukla R, Medeiros Silva J, Parmar A, Vermeulen BJ, Das S, Lucini Paioni A, Jekhmane S, Lorent J, Bonvin AM, Baldus M, Lelli M, Veldhuizen E, Breukink E, Singh I, Weingarth M (5 June 2020). "Mode of action of teixobactins in cellular membranes". Nature Communications. 11 (1): 2848. Bibcode:2020NatCo..11.2848S. doi:10.1038/s41467-020-16600-2. PMC 7275090. PMID 32503964.
- ↑ Azvolinsky, Anna. "New Antibiotic from Soil Bacteria". The Scientist. Retrieved 2 July 2015.
- ↑ Gallagher, James (7 January 2015). "Antibiotics: U.S. discovery labeled "game-changer" for medicine". BBC News. Retrieved 2 July 2015.
- ↑ Arias CA, Murray BE (2015). "A new antibiotic and the evolution of resistance". The New England Journal of Medicine. 372 (12): 1168–70. doi:10.1056/NEJMcibr1500292. PMC 4433155. PMID 25785976.
- ↑ Haridy March 25th, 2018, Rich (March 25, 2018). ""Game-changing" synthesized antibiotic successfully treats infections for the first time". newatlas.com. Retrieved 2018-04-03.
External links
- Dr. Kim Lewis, Northeastern University, speaks about Teixobactin during his seminar at the NIH entitled "New antibiotics from the microbial dark matter". February 15, 2017