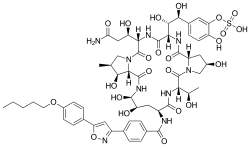
Micafungin
 | |
 | |
| Names | |
|---|---|
| Trade names | Mycamine |
IUPAC name
| |
| Clinical data | |
| Drug class | Antifungal (echinochandin)[1] |
| Main uses | Treat or prevent severe candidiasis[1] |
| Side effects | Low white blood cells, low red blood cells, low potassium, low calcium, nausea, liver problems, fever[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 99.8% |
| Metabolism | Via catechol-O-methyltransferase pathway |
| Elimination half-life | 11–17 hours |
| Excretion | 40% feces, <15% urine |
| Chemical and physical data | |
| Formula | C56H71N9O23S |
| Molar mass | 1270.28 g·mol−1 |
InChI
| |
Micafungin, sold under the brand name Mycamine, is an antifungal medication used to treat or prevent severe candidiasis.[1] It may also be used for aspergillosis.[2] It is given by gradual injection into a vein.[1]
Common side effects include low white blood cells, low red blood cells, low potassium, low calcium, nausea, liver problems, and fever.[1] Other side effects may include anaphylaxis and kidney problems.[2] Safety in pregnancy is unclear.[3] It is an echinochandin which stops the production of beta-1,3-glucan, an essential component of fungal cell walls.[1]
Micafungin was approved for medical use in the United States in 2005 and Europe in 2008.[2][1] It is available as a generic medication.[4] It is on the World Health Organization's List of Essential Medicines.[5] In the United States 7 doses of 100 mg costs about 370 USD.[4] This amount in the United Kingdom costs about £2,000.[6]
Medical uses
Micafungin is indicated for the treatment of candidemia, acute disseminated candidiasis, Candida peritonitis, abscesses and esophageal candidiasis. Since January 23, 2008, micafungin has been approved for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation (HSCT).
Dosage
It is used at a dose of 100 to 150 mg per day for active infection.[2] A dose of 50 mg per day may be used for prevention.[2]
Mechanism of action
Micafungin works by way of concentration-dependent inhibition of 1,3-beta-D-glucan synthase resulting in reduced formation of 1,3-beta-D-glucan, which is an essential polysaccharide comprising one-third of the majority of Candida spp. cell walls. This decreased glucan production leads to osmotic instability and thus cellular lysis. [7] [8]
Metabloism
The metabolism of micafungin occurs hepatically via acryp sulfatase followed by secondary metabolism by a transferase. Precautions should be taken with regards to dosing, as micafungin weakly inhibits CYP3A4.[9][10]
Society and culture
Micafungin is a natural antifungal product derived from other fungi as a defense mechanism for competition of nutrients, etc. To be specific, micafungin is derived from FR901379, and is produced by Coleophoma empetri.[11][12]
References
- 1 2 3 4 5 6 7 8 "Mycamine". Archived from the original on 10 March 2021. Retrieved 18 November 2021.
- 1 2 3 4 5 "Micafungin Monograph for Professionals". Drugs.com. Archived from the original on 24 July 2021. Retrieved 18 November 2021.
- ↑ "Micafungin (Mycamine) Use During Pregnancy". Drugs.com. Archived from the original on 23 January 2021. Retrieved 18 November 2021.
- 1 2 "Micafungin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 18 November 2021.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 632. ISBN 978-0857114105.
- ↑ Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, et al. (October 2007). "Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis". Clinical Infectious Diseases. 45 (7): 883–93. doi:10.1086/520980. PMID 17806055.
- ↑ Pettengell K, Mynhardt J, Kluyts T, Lau W, Facklam D, Buell D (August 2004). "Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agent". Alimentary Pharmacology & Therapeutics. 20 (4): 475–81. doi:10.1111/j.1365-2036.2004.02083.x. PMID 15298643. S2CID 31500007.
- ↑ Carver PL (October 2004). "Micafungin". The Annals of Pharmacotherapy. 38 (10): 1707–21. doi:10.1345/aph.1D301. PMID 15340133.
- ↑ Kohno S, Masaoka T, Yamaguchi H, Mori T, Urabe A, Ito A, et al. (2004). "A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan". Scandinavian Journal of Infectious Diseases. 36 (5): 372–9. doi:10.1080/00365540410020406. PMID 15287383. S2CID 10873612.
- ↑ Hashimoto S (January 2009). "Micafungin: a sulfated echinocandin". The Journal of Antibiotics. 62 (1): 27–35. doi:10.1038/ja.2008.3. PMID 19132058.
- ↑ Fujie A (2007). "Discovery of micafungin (FK463): A novel antifungal drug derived from a natural product lead". Pure and Applied Chemistry. 79 (4): 603–614. doi:10.1351/pac200779040603.
External links
- "Micafungin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-04-24. Retrieved 2021-10-15.
- Eschenauer G, Depestel DD, Carver PL (March 2007). "Comparison of echinocandin antifungals". Therapeutics and Clinical Risk Management. 3 (1): 71–97. doi:10.2147/tcrm.2007.3.1.71. PMC 1936290. PMID 18360617.
| Identifiers: |
|---|